Cd-MOF based on 4,4'-dicarboxydiphenyl ether and preparation method thereof
A technology of dicarboxydiphenyl ether and repeating unit is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc. The effect of wide source of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation of Cd-MOF based on 4,4'-dicarboxydiphenyl ether
[0040] The preparation method comprises the following steps:
[0041] (1) Dissolve 4,4'-dicarboxydiphenyl ether (0.063g, 0.3mmol) in 10mL water by heating, and adjust the pH value to 7-8 with NaOH;
[0042] (2) Dissolve dmbpy (0.028g, 0.15mmol) ligand in 5mL ethanol solution, then add Cd(NO 3 ) 2 4H 2 O (0.093g, 0.3mmol);
[0043] (3) Add the substances in steps 1 and 2 into a 23mL polytetrafluoroethylene reactor, keep at 130°C for 72 hours under autogenous pressure, and then cool down to room temperature at a rate of 5°C / h;
[0044] (4) The product obtained in step 3 was filtered, and the crystals were collected, washed with absolute ethanol and dried to obtain colorless crystals (calculated yield according to oba: 48%).
[0045] 2. Identification of Cd-MOF based on 4,4’-dicarboxydiphenyl ether
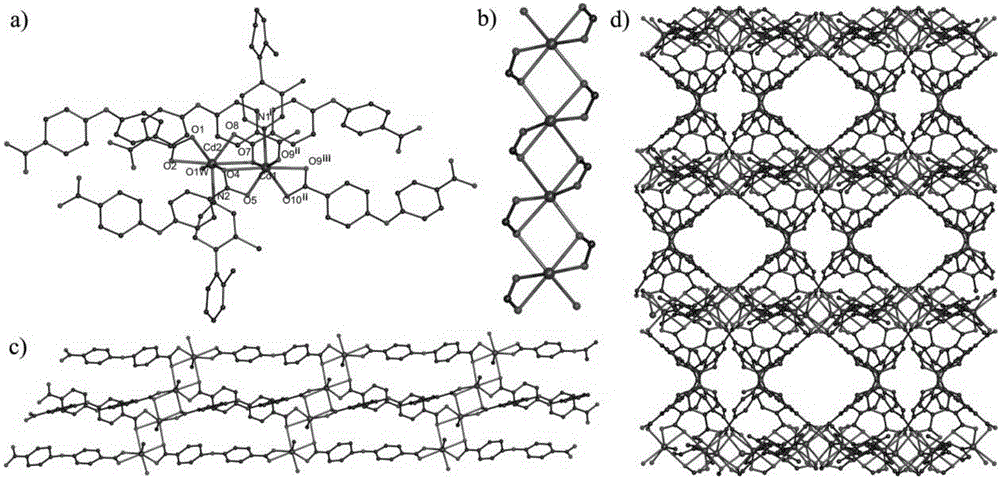
[0046] Through the following elemental analysis, IR spectrum and X-ray single crystal diffraction analys...
Embodiment 2
[0057] Embodiment 2: thermal stability and chemical stability experiment
[0058] In this example, the 4,4'-dicarboxydiphenyl ether-based Cd-MOF obtained in Example 1 was used to study thermal stability and chemical stability.
[0059] 1. Research on thermal stability of Cd-MOF based on 4,4'-dicarboxydiphenyl ether
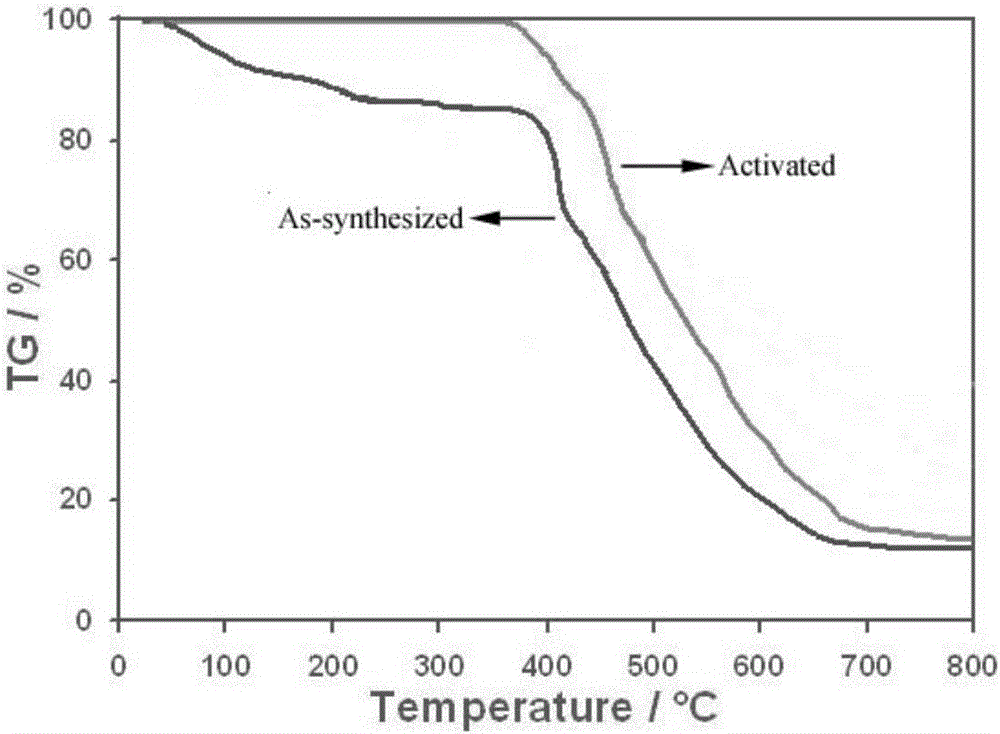
[0060] Weighed 3 mg of the prepared Cd-MOF and the activated Cd-MOF (vacuum dried at 130 °C for 12 h), and measured its thermal stability in the range of 25-800 °C at a heating rate of 10 °C / min.
[0061] Experimental results such as figure 2 Shown: Cd-MOF loses 2 ethanol molecules and 4 lattice water molecules in the coordination environment at about 200°C, and the mass loss is about 15.1%; at 200-250°C, one coordination water molecule is lost, The mass loss is about 1.75%; after about 380°C, the framework structure of Cd-MOF begins to decompose, and finally becomes the metal oxide CdO. The activated Cd-MOF can exist stably at 380°C.
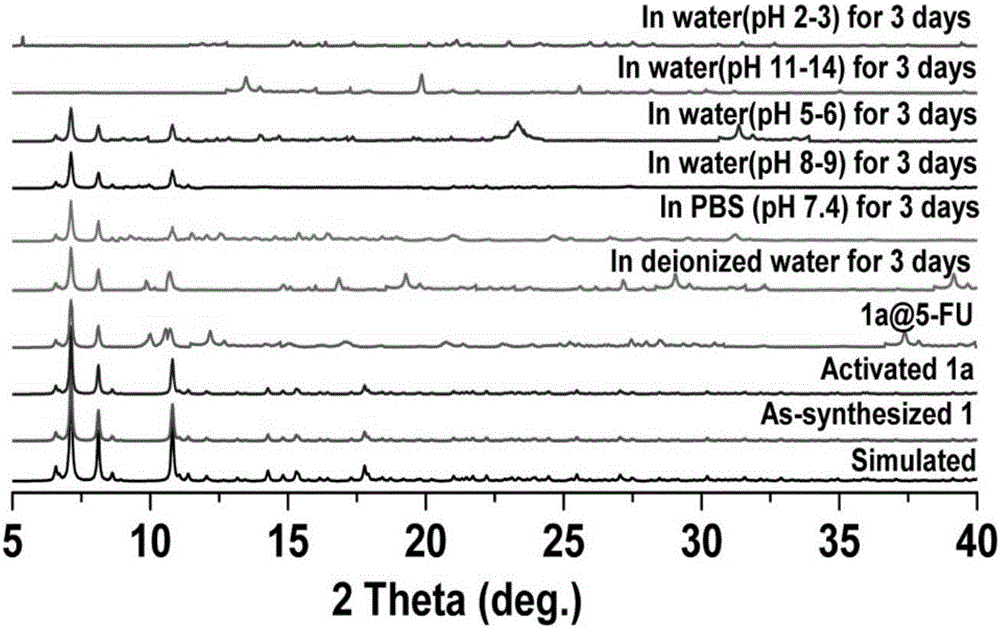
[0062] 2. Chemical sta...
Embodiment 3
[0069] In this example, the 4,4'-dicarboxydiphenyl ether-based Cd-MOF obtained in Example 1 was used to study the encapsulation and sustained release of 5-fluorouracil. The specific research methods are as follows:
[0070] 1. Determination of porosity of Cd-MOF based on 4,4'-dicarboxydiphenyl ether
[0071] At 77K, the activated Cd-MOF and the Cd-MOF after 3 days of water immersion and 5-Fu loading were measured against N2 isothermal adsorption.
[0072] N 2 The adsorption isotherm shows a type I characteristic of the microporous material ( Figure 5 a), through theoretical calculation, the specific surface area of the pores of the activated Cd-MOF is 775m 2 g -1 . At the same time, the specific surface area of Cd-MOF remained basically unchanged after being immersed in water for 3 days (775-740m 2 g -1 ), so the porous structure of Cd-MOF is stable in water, indicating that the hydrophobic group methyl group in the Cd-MOF framework is very close to the cadmium met...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com