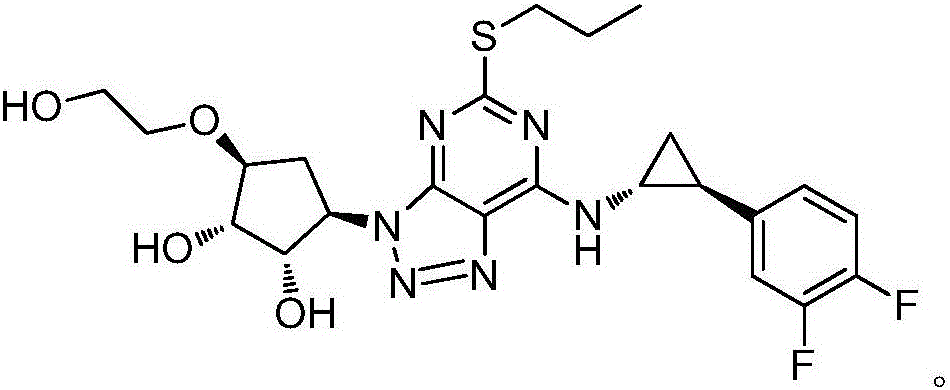
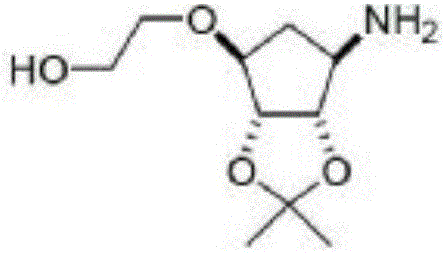
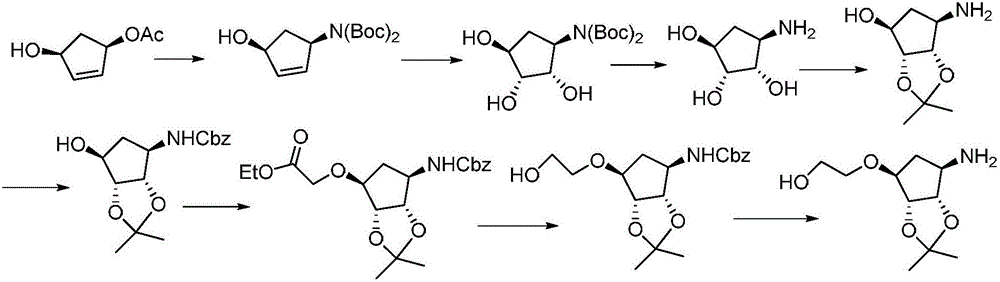
Synthesis process of ticagrelor intermediate
A synthesis process, the technology of ticagrelor, applied in the field of synthesis process of ticagrelor intermediates, can solve the problems of no stereoselectivity, expensive raw materials, long steps, etc. Short, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of compounds shown in formula I
[0034] In a 150ml flask, 1.36g (10mmol) ZnCl 2 , 12.11g (100mmol) benzaldehyde oxime, 6.28g (95mmol) cyclopentadiene were added to 60ml 1,4-dioxane, stirred and reacted at 40°C for 6 hours, the reaction was completed, the reaction solution was concentrated, poured into water for cleaning , filtered, and then recrystallized from petroleum ether to obtain 14.75 g of the compound shown in formula I, with a yield of 82.9% and a purity of 99.32%.
Embodiment 2
[0036] Synthesis of compounds shown in formula I
[0037] In a 150ml flask, 2.72g (20mmol) ZnCl 2 , 12.11g (100mmol) benzaldehyde oxime, 5.95g (90mmol) cyclopentadiene were added to 60ml 1,4-dioxane, stirred and reacted at 20°C for 4 hours, the reaction was completed, the reaction solution was concentrated, poured into water for cleaning , filtered, and then recrystallized from petroleum ether to obtain 13.45 g of the compound shown in formula I, with a yield of 79.8% and a purity of 98.97%.
Embodiment 3
[0039] Synthesis of compounds shown in formula I
[0040]In a 150ml flask, 1.33g (10mmol) AlCl 3 , 12.11g (100mmol) benzaldehyde oxime, 5.29g (80mmol) cyclopentadiene were added to 60ml 1,4-dioxane, stirred and reacted at 30°C for 4 hours, the reaction was completed, the reaction solution was concentrated, poured into water for cleaning Three times, filtered, and then recrystallized from petroleum ether to obtain 12.24 g of the compound represented by formula I, with a yield of 81.7% and a purity of 99.03%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com