A method of preparing micro-powder-type capecitabine
A capecitabine and micropowder technology, which is applied in the field of preparing micropowder capecitabine, can solve problems such as unfavorable industrial production, difficult manual control, and inability to achieve, and achieves convenient preparation granulation, improved dissolution, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Put 150g of capecitabine crude product and 300ml of tetrahydrofuran into a 1000ml reaction flask, dissolve at 25℃, add 600ml methyl tert-butyl ether dropwise with stirring, stop stirring after addition, keep for 1.5 hours for crystallization, stir and cool to -10℃ , Suction filtration, and blast drying to obtain 137 g of micronized capecitabine, with a yield of 91.4%, a purity of 99.89%, and a melting point of 117.5-119°C.
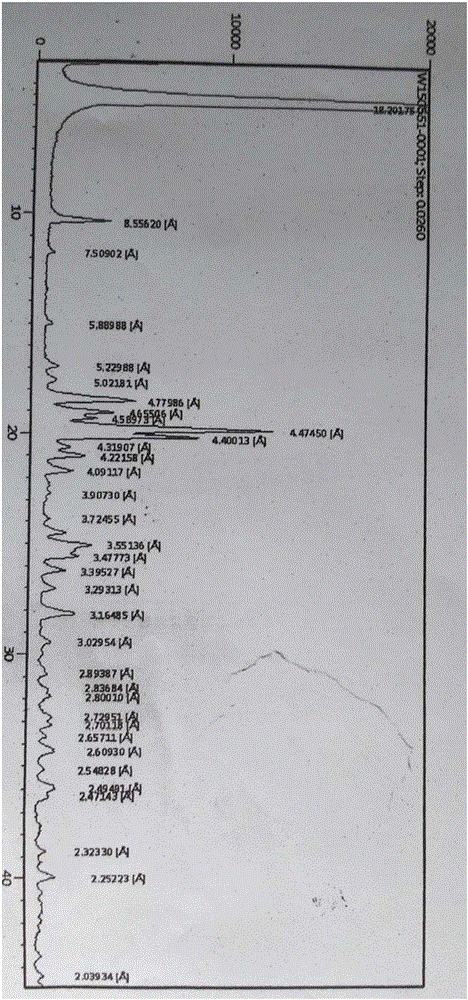
[0032] The obtained micronized capecitabine was measured by X-ray powder diffraction pattern, using Cu-Kα radiation, the X-ray powder diffraction expressed in 2θ angles was at 4.9°±0.2°, 10.3°±0.2°, 11.7°±0.2 °, 15.0°±0.2°, 17.0°±0.2°, 17.6°±0.2°, 18.6°±0.2°, 19.1°±0.2°, 19.4°±0.2°, 19.8°±0.2°, 20.2°±0.2°, 20.5°±0.2°, 21.0°±0.2°, 21.7°±0.2°, 22.7°±0.2°, 23.9°±0.2°, 25.0°±0.2°, 25.6°±0.2°, 26.2°±0.2°, 27.1° ±0.2°, 28.2°±0.2°, 29.5°±0.2°, 30.8°±0.2°, 31.5°±0.2°, 31.9°±0.2°, 32.8°±0.2°, 33.1°±0.2°, 33.7°±0.2 °, 34.3°±0.2°, 32.5°±0.2°, 35.9°±0.2°, 36.3°±...
Embodiment 2
[0034] Put 150g of capecitabine crude product and 150ml of tetrahydrofuran into a 1000ml reaction flask, dissolve at 25℃, add 300ml methyl tert-butyl ether dropwise with stirring, stop stirring after addition, keep for 1.5 hours for crystallization, stir and cool to -10℃ , Suction filtration, and blast drying to obtain 138 g of micronized capecitabine, with a yield of 92.0%, a purity of 99.8%, and a melting point of 117-119°C.
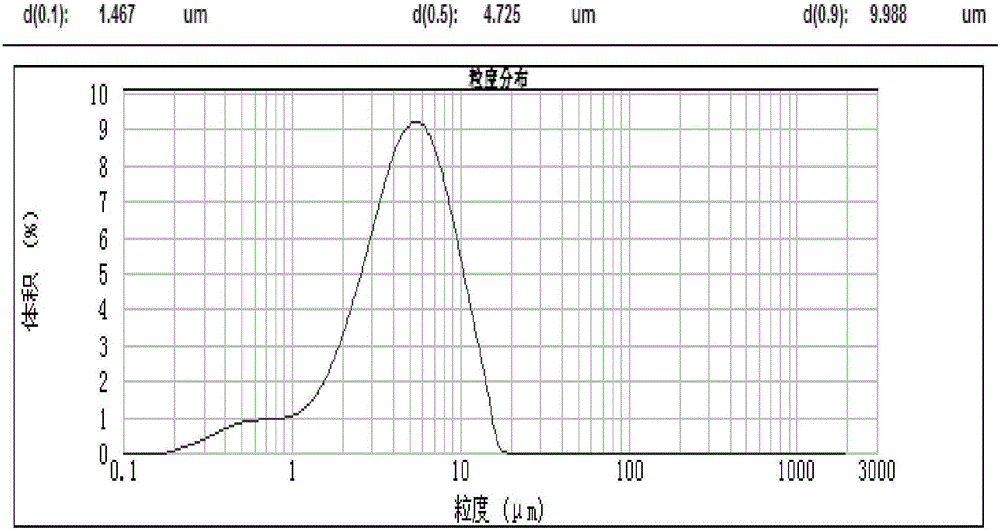
[0035] The obtained micronized capecitabine was measured by a laser particle size analyzer, and its particle size was: d(0.1)=1.403μm, d(0.5)=4.565μm, d(0.9)=9.645μm, the result is as follows Figure 4 Shown.
[0036] The capecitabine prepared in Examples 1 to 2 was prepared with reference to the Xeloda prescription that has been marketed to prepare capecitabine tablets. The in vitro dissolution test method: Take this product according to the second method of Annex XC of the 2010 edition of the Chinese Pharmacopoeia. Purified water, pH 1.2 hydrochloric acid...
Embodiment 3
[0038] Put 150g of crude capecitabine and 300ml of tetrahydrofuran into a 1000ml reaction flask, dissolve at 30°C, add 600ml methyl tert-butyl ether dropwise with stirring, stop stirring after addition, keep crystallization for 1.5 hours, stir and cool to -10°C , Suction filtration, and blast drying to obtain 136.4 g of micronized capecitabine, with a yield of 90.9%, a purity of 99.87%, and a melting point of 118-119°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com