Preparation and application of triterpene drug for inhibiting synthesis and secretion of apoB48 in intestinal tracts
A technology of triterpene compounds and drugs, which can be applied in the direction of drug combinations, organic active ingredients, and medical preparations containing active ingredients, and can solve problems such as limited development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of Cyclocarya triterpenoids
[0025] 48.5 kg of dried Cyclocarya paliurus leaves were heated and refluxed with 80% ethanol for 3 times, each time for 2 hours, the extracts were combined, concentrated under reduced pressure until there was no alcohol smell, the residue was suspended with water, and then washed with chloroform, ethyl acetate After extraction, the solvent was recovered separately to obtain the chloroform fraction (3.65kg), the ethyl acetate fraction (120g) and the remaining water fraction.
[0026] Take 3kg of the extract from the chloroform part, go through silica gel column chromatography under reduced pressure, and use chloroform-methanol (100:0, 100:5, 5:1, 0:100) gradient elution, and each gradient is combined together to obtain 4 fractions (Fr. 1-4). Fr.2 (400g) was subjected to silica gel column chromatography, eluted with a gradient of chloroform-methanol (100:3→0:100), checked by TLC, and combined to obtain 4 sub-fractions (...
Embodiment 2
[0027] Example 2 Compound structure identification data
[0028] 50 3β, 23-dihydroxy-1, 12-dioxo-oleanolic acid (3β, 23-dihydroxy-1, 12-dioxo-olean-28-oic acid)
[0029] White powder (methanol), mp 287~289℃, (c 0.095MeOH), easily soluble in chloroform, methanol, acetonitrile and other organic solvents, Liebermann-Burchard reaction is positive, vanillin-concentrated sulfuric acid shows blue-purple. IR(KBr)cm -1 : 3441, 2946, 1696, 1050; UVλ max (CH 3 OH): 203nm. HR-TOF-MS m / z: 501.3218[M-H] - (C 30 h 45 o 6 , calcd.501.3216), the molecular formula is C 30 h 46 o 6 , with a molecular weight of 502. The UV spectrum shows a maximum absorption peak at . The IR spectrum shows a strong hydroxyl absorption peak (cm -1 ), C-H bond stretching vibration absorption peak (cm -1 ), carbonyl absorption peak (cm -1 ) and C-O bond stretching vibration absorption peaks (cm -1 ). 1 H NMR (C 5 D. 5 N, 600MHz) and 13 C NMR (C 5 D. 5N, 125MHz) data are shown in Table 1.
[...
Embodiment 3
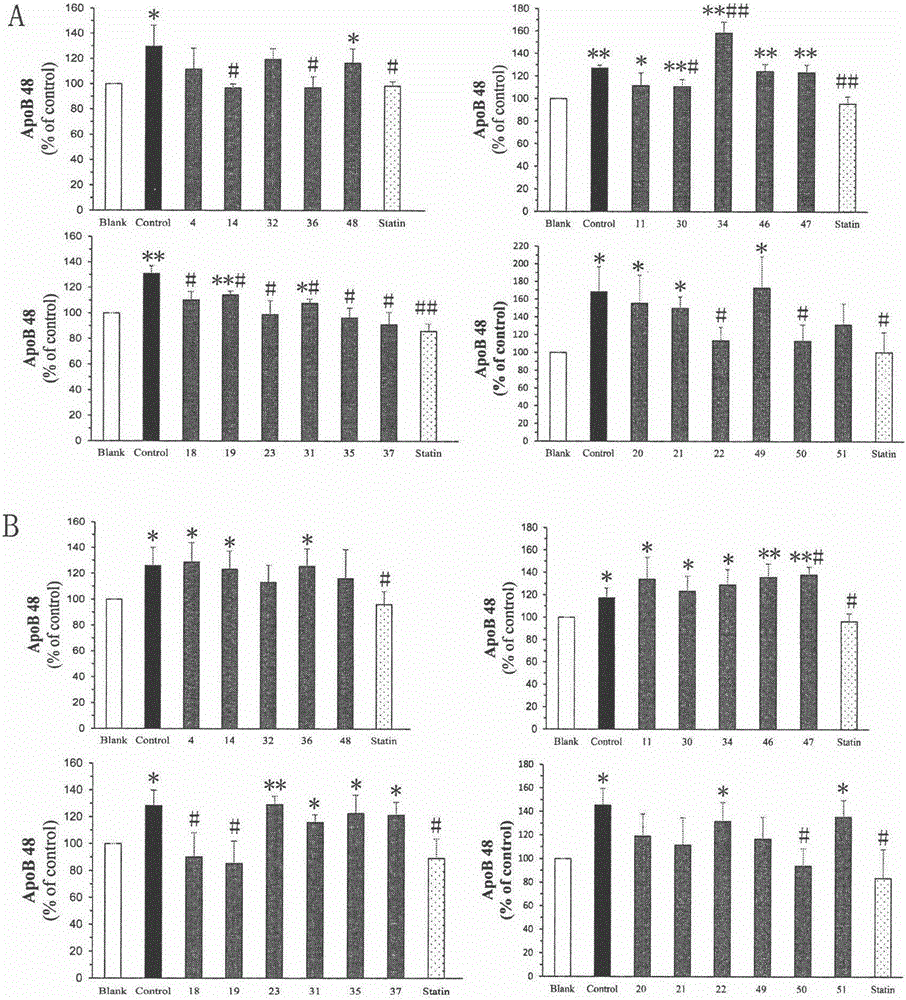
[0045] Example 3 Cyclocarya triterpenoids regulate the activity of Caco-2 cells secreting apoB48
[0046] 1. Materials
[0047] 1.1 Test article
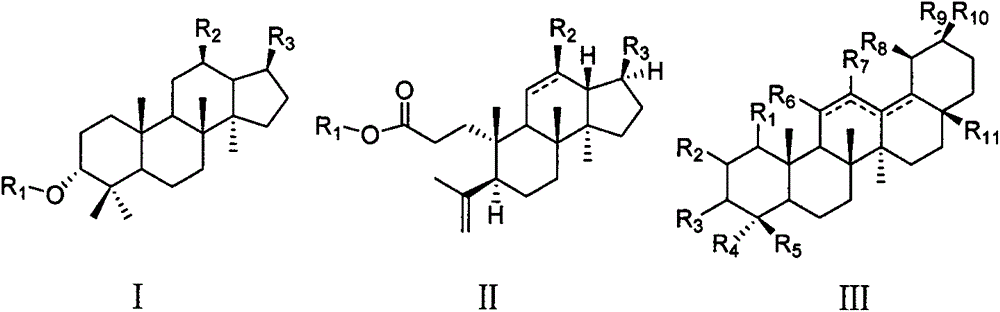
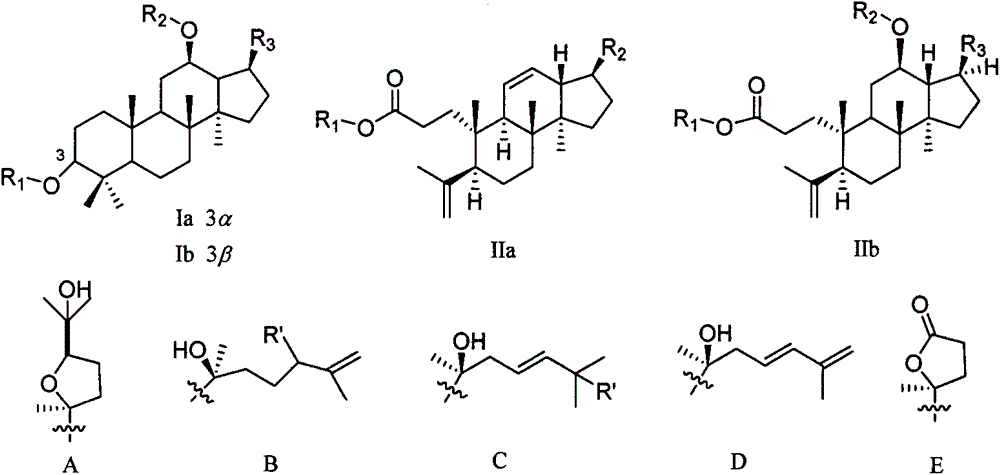
[0048] The test samples are 22 triterpenoids isolated from Cyclocarya paliurus, numbered 4, 11, 14, 18-23, 30-32, 34-37 and 46-51, and the structural formulas are as described above. Stock solution of the test product: take 1 mg of the triterpene compound, dissolve it in 100 μL of dimethyl sulfoxide (DMSO), and then dilute it 10 times with PBS, store it at 4°C until use.
[0049] 1.2 Drugs and reagents
[0050] Caco-2 cell line was purchased from Nanjing Kaiji Biotechnology Development Co., Ltd. (batch number 20151019).
[0051] Table 4 Experimental reagents and drugs
[0052]
[0053] 2. Experimental method
[0054] 2.1 Establishment of Caco-2 cell model
[0055] (1) Recovery of Caco-2 cells
[0056] Thaw the previously frozen Caco-2 cells in warm water at 37°C, dilute with complete DMEM high-glucose medium containing 10%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com