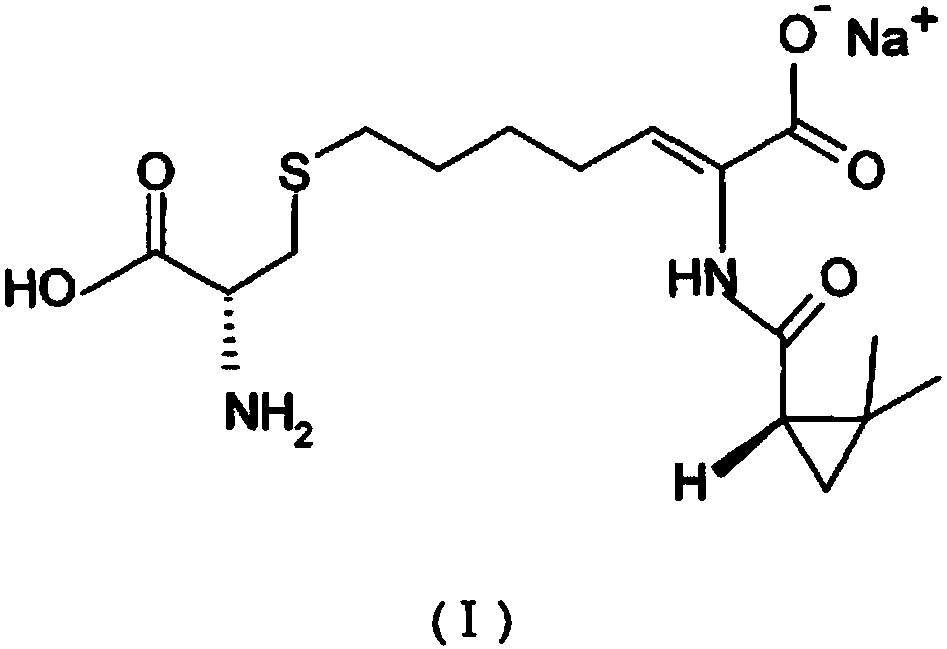
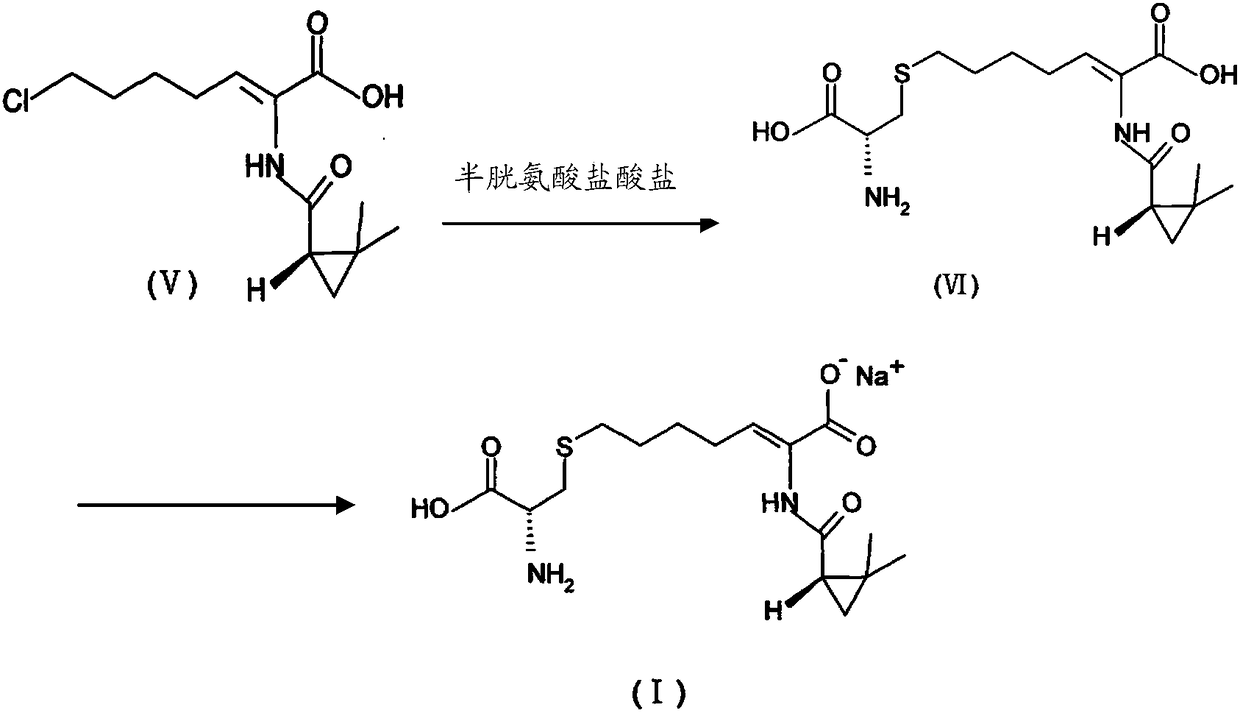
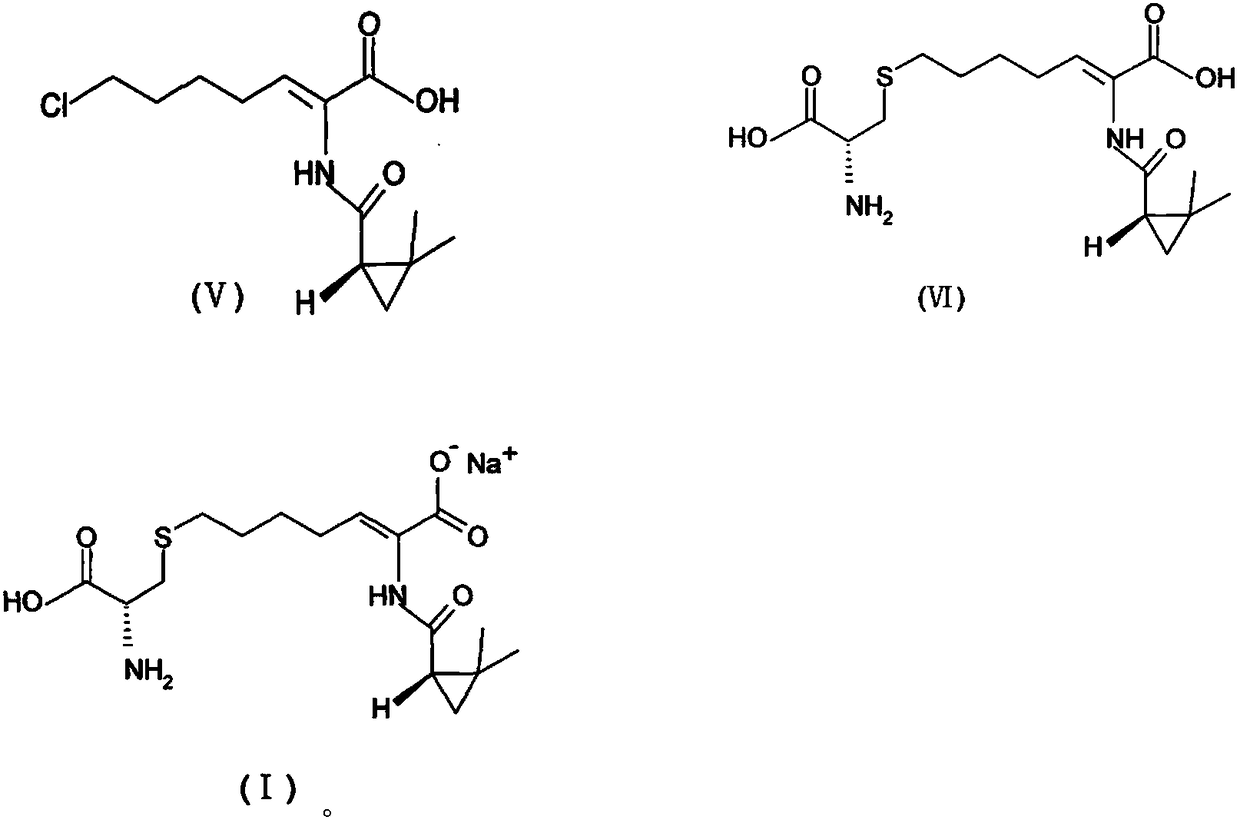
A kind of preparation method of cilastatin sodium crude drug
A technology of cilastatin sodium and cilastatin, which is applied in the field of medicinal chemistry, can solve the problems of taking a long time, reducing the activity of raw materials, and consuming a long time, so as to avoid the reduction of chemical activity and improve the quality and yield , to avoid the effect of excessive losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1: the preparation of cilastatin sodium of the present invention
[0087] (1) Preparation of compound cilastatin shown in formula (VI):
[0088] At 0°C, add 15g of sodium hydroxide to 100g of water, stir for 10min, then add 10g of compound (Z)-7-chloro-2((S)-2,2-dimethylcyclo Propyl formamido)-2-heptenoic acid, stirred for 10min; at -5°C, 9g of cysteine hydrochloride monohydrate was added, stirred for 10min, and the reaction started at 25°C until analyzed by HPLC, Complete the reaction when the normalized content of (Z)-7-chloro-2((S)-2,2-dimethylcyclopropylcarboxamido)-2-heptenoic acid in the resulting reaction solution is less than 5%. After cooling down to 25°C, add 300g of water to the solution, stir, and slowly add 6M hydrochloric acid solution to the solution to adjust the pH value to 1. Add the obtained solution to a Φ60*5.5m macroporous adsorption resin column HZ-820, first elute with water, and then change to acetone aqueous solution for elution...
Embodiment 2
[0091] Embodiment 2: the preparation of cilastatin sodium of the present invention
[0092] (1) Preparation of compound cilastatin shown in formula (VI):
[0093] At 10°C, add 9g of sodium hydroxide to 80g of water, stir for 10min, then add 10g of compound (Z)-7-chloro-2((S)-2,2-dimethylcyclo Propyl formamido)-2-heptenoic acid, stirred for 10min; at 10°C, 8.5g of cysteine hydrochloride monohydrate was added, stirred for 10min, and the reaction started at 35°C until it was analyzed by HPLC The reaction is completed when the normalized content of (Z)-7-chloro-2((S)-2,2-dimethylcyclopropylcarboxamido)-2-heptenoic acid in the obtained reaction solution is less than 5%; After cooling down to 30° C., 250 g of water was added to the solution, stirred, and 6M hydrochloric acid solution was slowly added to the solution to adjust the pH value to 1.5. Add the obtained solution to a Φ35*3.5m macroporous adsorption resin column HZ-818, first elute with water, and then change to metha...
Embodiment 3
[0096] Embodiment 3: the preparation of cilastatin sodium of the present invention
[0097] (1) Preparation of compound cilastatin shown in formula (VI):
[0098] At 0°C, add 11g of sodium hydroxide to 70g of water, stir for 10min, then add 10g of compound (Z)-7-chloro-2((S)-2,2-dimethylcyclo Propyl formamido)-2-heptenoic acid, stirred for 10min; at 0°C, 8.3g of cysteine hydrochloride monohydrate was added, stirred for 10min, and the reaction started at 45°C until it was analyzed by HPLC The reaction is completed when the normalized content of (Z)-7-chloro-2((S)-2,2-dimethylcyclopropylcarboxamido)-2-heptenoic acid in the obtained reaction solution is less than 5%; After cooling down to 5° C., 200 g of water was added to the solution, stirred, and 6M hydrochloric acid solution was slowly added to the solution to adjust the pH value to 2. Add the obtained solution to a Φ20*2.5m macroporous adsorption resin column HZ-816, first elute with water, and then change to methanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com