Triamcinolone acetonide acetate crystal form B, preparation method of triamcinolone acetonide acetate crystal form B, medicinal composition containing crystal form B and application of crystal form B
A technology of triamcinolone acetonide acetate and its composition, which is applied in the field of new crystal form B of triamcinolone acetonide acetate and its preparation, can solve the problems of patients' pain, unqualified products, and easy caking, and achieve good reproducibility, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Mix 12 g of triamcinolone acetonide acetate in 500 mL of methanol, add a magnetic stirrer and stir at 200 rpm, and it can be completely dissolved at 50°C. Continue to stir and cool to 5°C to obtain a suspension, which is filtered and dried under reduced pressure at room temperature. The crystal form B was obtained as 11.4 g of white crystalline powder, and the yield was 95%.
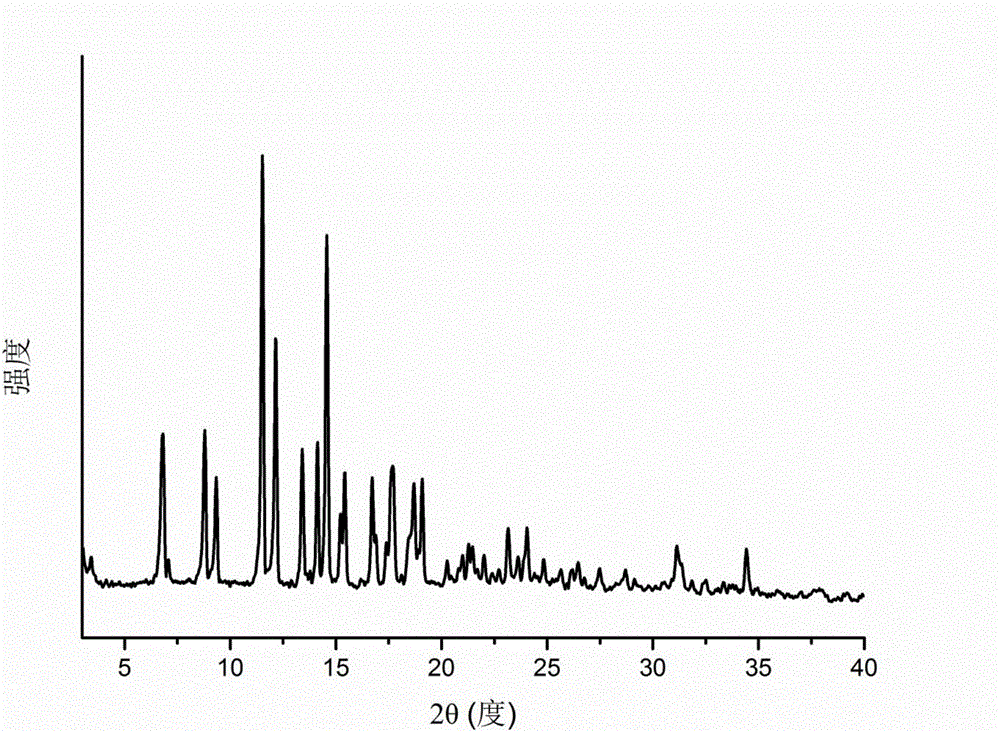
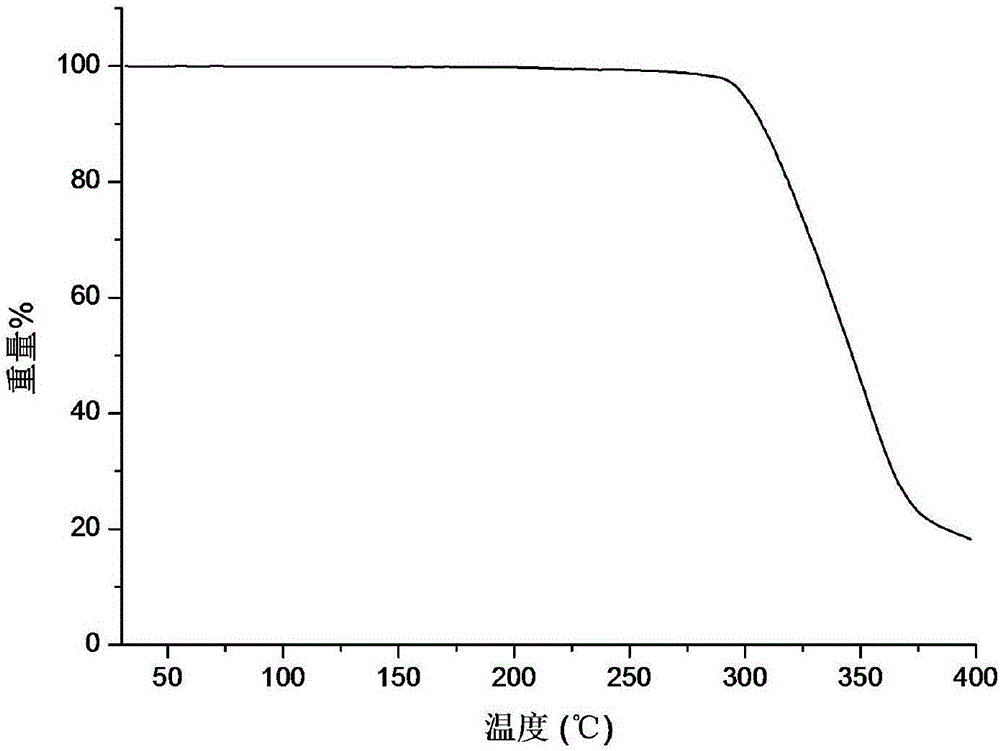
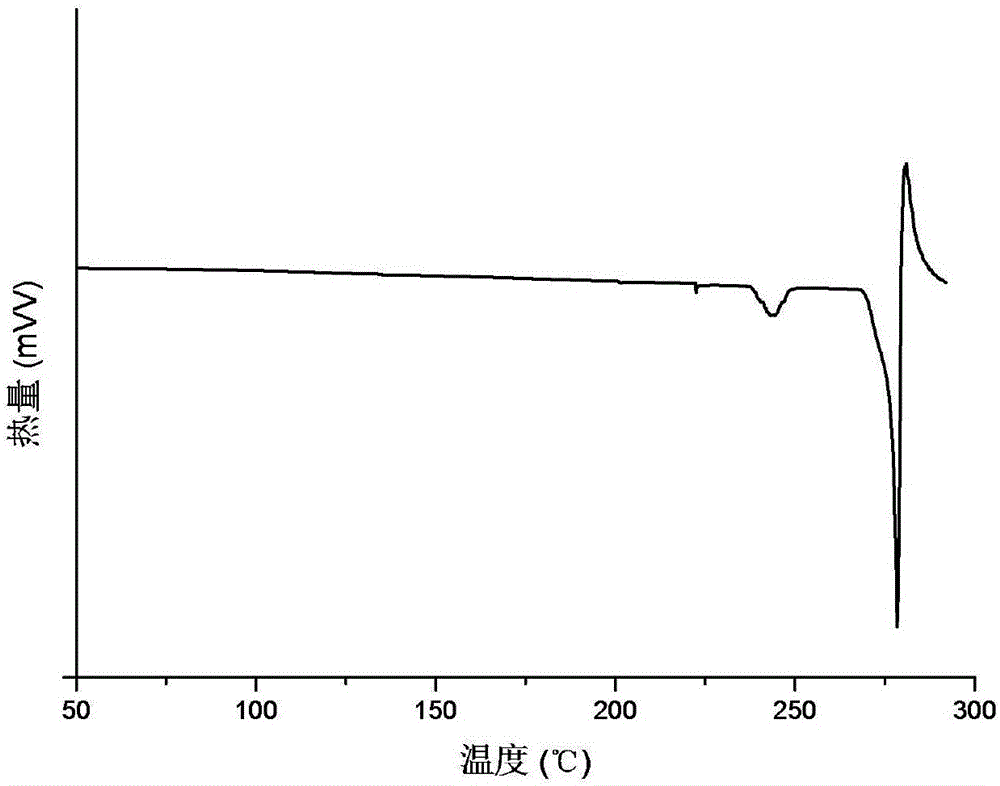
[0039] The X-ray powder diffraction (XRPD) pattern of the crystal form B of triamcinolone acetonide acetate of embodiment 1 is shown in figure 1 ; Thermogravimetric analysis (TG) diagram see figure 2 ; Differential scanning calorimetry (DSC) diagram see image 3 ; Infrared spectrum (IR) figure see Figure 4 ; Commercially available triamcinolone acetonide acetate crystal form and the contrast figure of crystal form B of the present invention are as follows Figure 5 shown. Scanning electron microscope (SEM) picture see Figure 6 The crystal form B is suspended in water, and the scanning ele...
Embodiment 2
[0042] Mix 10 g of triamcinolone acetonide acetate in 500 mL of ethanol, add a magnetic stirring bar to stir at 200 rpm, and heat to 60° C. to dissolve completely. Continue to stir and cool to 5°C to obtain a suspension, which is filtered and dried under reduced pressure at room temperature. 9.4 g of white crystals were obtained as crystal form B, and the yield was 94%.
Embodiment 3
[0044] Mix 15 g of triamcinolone acetonide acetate in 500 mL of methyl ethyl ketone, add a magnetic stirring bar to stir at 200 rpm, and heat to 70° C. to dissolve completely. Continue to stir and cool to 5°C to obtain a suspension, which is filtered and dried under reduced pressure at room temperature. The crystal form B was obtained as 12.4 g of white crystalline powder, and the yield was 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com