Preparing method of 11alpha-hydroxy-16alpha, 17alpha-epoxy progesterone dehydrogenation substance
The technology of epoxy progesterone and hydroxyl group is applied in the field of preparation of 11α-hydroxy-16α, 17α-epoxyprogesterone dehydrogenate, and can solve the problems of difficulty in effective contact of biological enzymes, influence and high process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
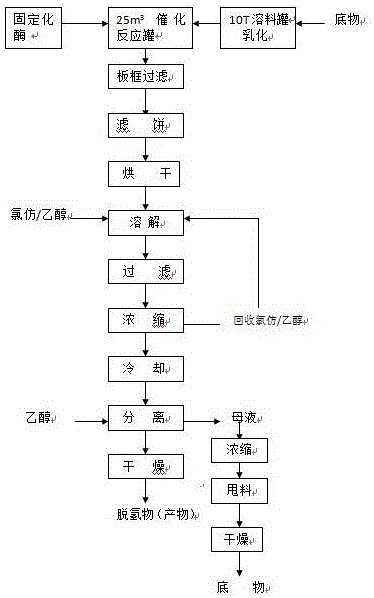
[0021] This example provides the preparation method of 11α-hydroxyl-16α, 17α-epoxy progesterone dehydrogenate, the process flow chart of the preparation method is as follows figure 1 As shown, the method specifically includes the following steps:
[0022] 1. Preparation of toluene-tolerant Ochra
[0023] First, inoculate the strain of Ochraus ochrax test tubes on the potato slant medium, culture at 28°C for 7 days to generate yellow spores, then transfer the yellow spores to the slant of the yeast extract slant medium, and cultivate at 28°C for 7 days to obtain the production Use the slant; then use sterile water to rinse the production slant to obtain a spore suspension, and control the concentration of the spore suspension to be 8×10 6 each / ml; wherein, the potato slant medium includes: potato 200 g / L, glucose 20 g / L, agar 20 g / L, yeast extract 1 g / L, sterile water 1000 ml, and the potato The pH value of the slant medium is 6.8;
[0024] Then the spore suspension was inse...
Embodiment 2
[0032] This example provides a method for preparing 11α-hydroxyl-16α, 17α-epoxyprogesterone dehydrogenate. The specific preparation steps are roughly the same as those in Example 1, except that in this example,
[0033] In the conversion step in the two-phase system, the inoculum of the toluene-tolerant Ochratos is controlled to account for 7% of the water / toluene two-phase fermentation solution; 1g of the 11α-Hydroxy-16α,17α-epoxyprogesterone.
[0034] In this example, after the water / toluene two-phase fermentation solution was catalyzed by microorganisms for 30 hours, the 11α-hydroxyl-16α,17α-ring in the water / toluene two-phase fermentation solution was analyzed by high-performance liquid chromatography (HPLC). The conversion rate of oxyprogesterone to 11α-hydroxy-16α, 17α-epoxy progesterone dehydrogenation is 98.7%; The recovery yield of the product was 90%, and the purity was 91%. The recovery rate of 11α-hydroxyl-16α, 17α-epoxy progesterone was 10%, and the purity was 98...
Embodiment 3
[0036] This example provides a method for preparing 11α-hydroxyl-16α, 17α-epoxy progesterone dehydrogenate, the specific preparation steps are roughly the same as those in Example 1, the difference is that in the two In the conversion step in the phase system, the inoculum amount of the toluene-tolerant ochratos is controlled to account for 8% of the water / toluene two-phase fermentation solution; 1.5g of the 11α-hydroxyl is added in every 100 ml of the fermentation broth -16α,17α-epoxyprogesterone.
[0037] The water / toluene two-phase fermentation solution described in this example was subjected to microbial catalysis for 20 hours, and analyzed by high performance liquid chromatography (HPLC), the 11α-hydroxyl-16α, 17α-epoxy progesterone The conversion rate of 11α-hydroxyl-16α, 17α-epoxy progesterone dehydrogenation was 98.6%; The yield was 90.5%, and the purity was 91%. The recovery of 11α-hydroxy-16α, 17α-epoxyprogesterone was 11%, and the purity was 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com