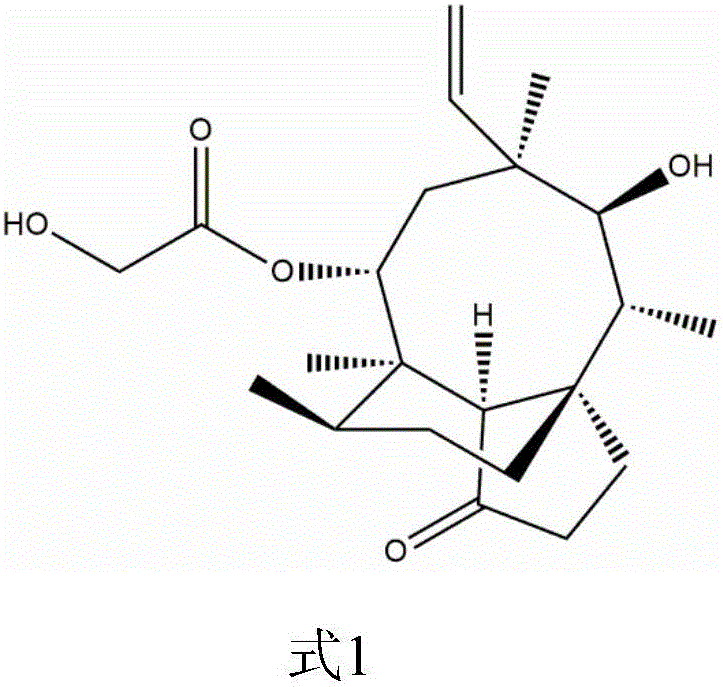
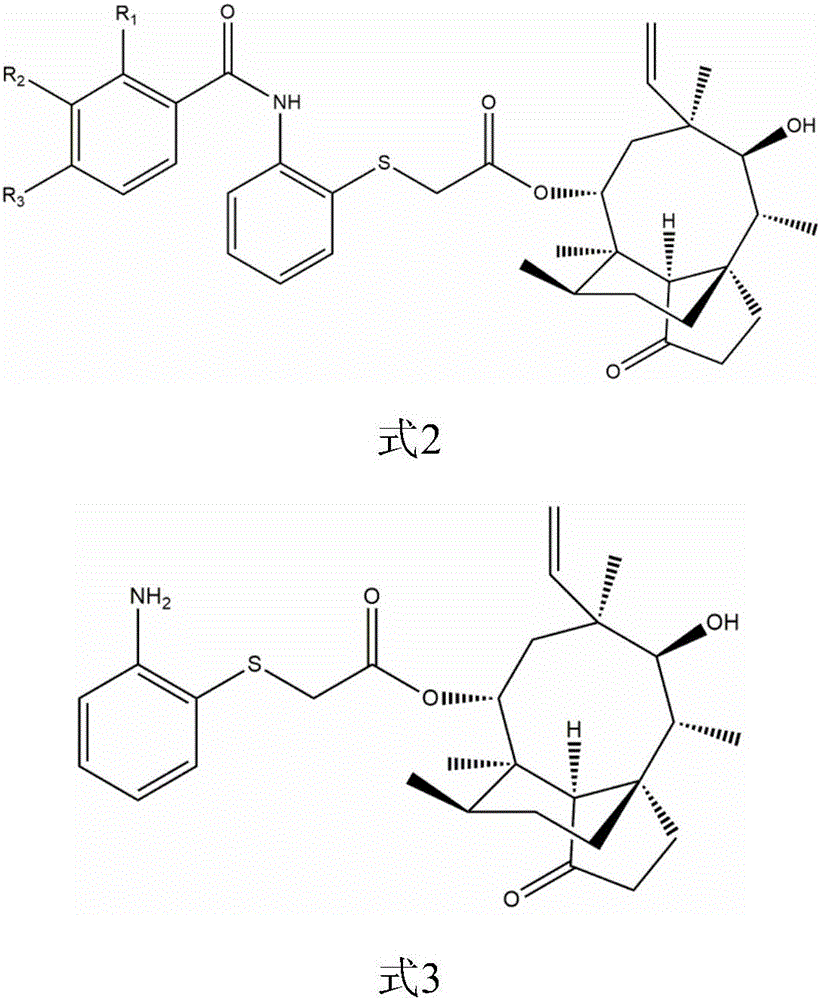
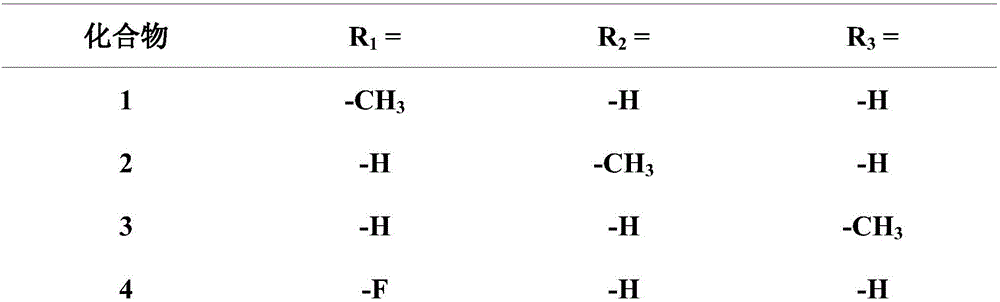
Pleuromutilin derivative with 2-amino phenyl mercaptan side chain and preparing method and application of pleuromutilin derivative
A technology of pleuromutilin and aminophenylthiol, which is applied in thioether preparation, sulfonate preparation, organic chemistry, etc., can solve the problem of rare drug-resistant bacteria, and achieve the effect of good in vitro antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Synthesis of 22-O-[2-(2-methylbenzamide)phenyl]thioacetyl Murelin (Compound 1)
[0062] Intermediate II 0.82g (1.88mmol) was dissolved in 35ml ethyl acetate, 2-methylbenzoic acid (2.07mmol) and oxalyl chloride 2.07mmol were added, heated and stirred at about 70°C for 1 hour to obtain the target product. The resulting mixed solution was evaporated to dryness by rotating to make the mixture redissolved in dichloromethane. Add 1g of 100-200 mesh silica gel and mix thoroughly. After the solvent evaporates, the above crude product-silica powder mixture is purified by column chromatography (200~300 The mesh silica gel powder is the stationary phase, and petroleum ether: ethyl acetate=1:1 is the mobile phase) to obtain the product 22-O-[2-(2-methylbenzamide)phenyl]thioacetyl murine ( The pure product of compound 1). The yield was 86.55%. HR-MS(ESI): Cal: 604.3091; Found: 604.3117.
Embodiment 2
[0063] Example 2: Synthesis of 22-O-[2-(3-methylbenzamido)phenyl]thioacetylmurine (Compound 2)
[0064] Intermediate II 0.82g (1.88mmol) was dissolved in 35ml of dichloromethane, 3-methylbenzoic acid (2.07mmol) and tert-butyl chloroformate 2.07mmol were added, heated and stirred at about 70°C for 1 hour to obtain the target product. The resulting mixed solution was evaporated to dryness by rotating to make the mixture redissolved in dichloromethane. Add 1g of 100-200 mesh silica gel and mix thoroughly. After the solvent evaporates, the above crude product-silica powder mixture is purified by column chromatography (200~300 The mesh silica gel powder is the stationary phase, and petroleum ether: ethyl acetate=1:1 is the mobile phase) to obtain the product 22-O-[2-(3-methylbenzamide)phenyl]thioacetyl Murelin ( Compound 2) pure product. The yield was 85.75%. HR-MS(ESI): Cal: 604.3091; Found: 604.3110.
Embodiment 3
[0065] Example 3: Synthesis of 22-O-[2-(4-methylbenzamido)phenyl]thioacetylmurine (Compound 3)
[0066] 0.82g (1.88mmol) of Intermediate II was dissolved in 35ml of dichloromethane, 4-methylbenzoic acid (2.07mmol) and 2.07mmol of thionyl chloride were added, heated and stirred at about 70°C for 1 hour to obtain the target product. The resulting mixed solution was evaporated to dryness by rotating to make the mixture redissolved in dichloromethane. Add 1g of 100-200 mesh silica gel and mix thoroughly. After the solvent evaporates, the above crude product-silica powder mixture is purified by column chromatography (200~300 The mesh silica gel powder is the stationary phase, and petroleum ether: ethyl acetate=1:1 is the mobile phase) to obtain the product 22-O-[2-(4-methylbenzamide)ethyl]thioacetyl murine ( Compound 3) pure product. The yield was 83.09%. HR-MS(ESI): Cal: 604.3091; Found: 604.3110.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com