Method for preparing 2, 5-furandicarboxylic acid by conducting catalytic oxidation on 5-hydroxymethylfurfural
A technology of hydroxymethylfurfural and furandicarboxylic acid, which is applied in the field of preparation of 2,5-furandicarboxylic acid, can solve the problems of low FDCA yield, poor stability, and only 4.2%, achieving high yield and selectivity, and reaction Mild conditions and good reusability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
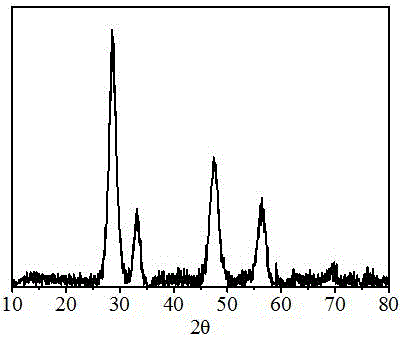
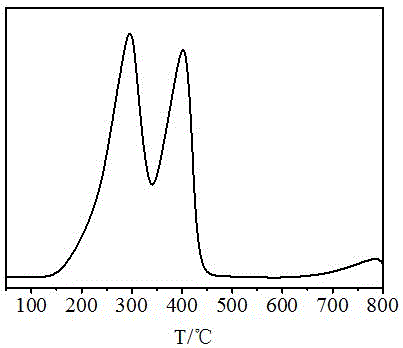
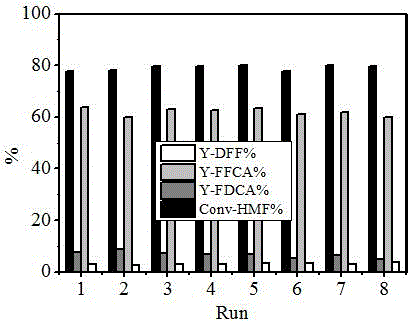
Image
Examples
Embodiment 1
[0025] Put 600mg of cerium-chromium composite oxide catalyst prepared by co-precipitation method (the mass percentage of cerium oxide is 50%), 10mL of 0.05mol / L HMF aqueous solution, 0.424g of sodium carbonate, into a stainless steel autoclave, and fill it with 3MPa oxygen As an oxygen source, it was reacted at 150° C. for 12 hours while being magnetically stirred. Finally, the reaction solution was analyzed by HPLC for substrate conversion rate and product yield. The conversion rate of HMF was 97.7%, and the yield of FDCA was 86.7%.
Embodiment 2
[0027] Put 100mg of cerium-manganese composite oxide catalyst prepared by hydrothermal method (the mass percentage of cerium oxide is 30%), 10mL of 0.05mol / L HMF aqueous solution, 0.08g of sodium hydroxide, into a stainless steel autoclave, and fill it with a 2MPa Oxygen was used as an oxygen source, and the reaction was carried out at 110° C. for 8 hours while magnetically stirring. Finally, the reaction solution was analyzed by HPLC for substrate conversion rate and product yield. The conversion rate of HMF was 98.5%, and the yield of FDCA was 81.8%.
Embodiment 3
[0029] Add 30mg of cerium-iron composite oxide catalyst prepared by sol-gel method (the mass percentage of cerium oxide is 10%), 10mL of 0.05mol / L HMF aqueous solution, 0.015g of ethylenediamine, into a stainless steel autoclave, and fill it with 0.5 MPa oxygen was used as an oxygen source, and the reaction was carried out at 70° C. for 6 hours while magnetically stirring. Finally, the reaction solution was analyzed by HPLC for substrate conversion rate and product yield. The conversion rate of HMF was 98.6%, and the yield of FDCA was 84.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com