Antibacterial acellular dermal dressing with vascularization inducing capability and preparation method thereof
A decellularized dermis and vascularization technology, applied in medical science, prosthesis, etc., can solve the problems of wound infection, cross-infection risk, and limited application, and achieve the effect of improving the ability of vascularization and promoting the proliferation of endothelial cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
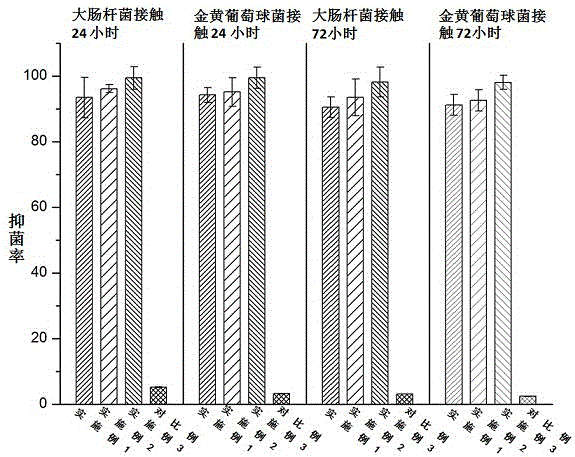
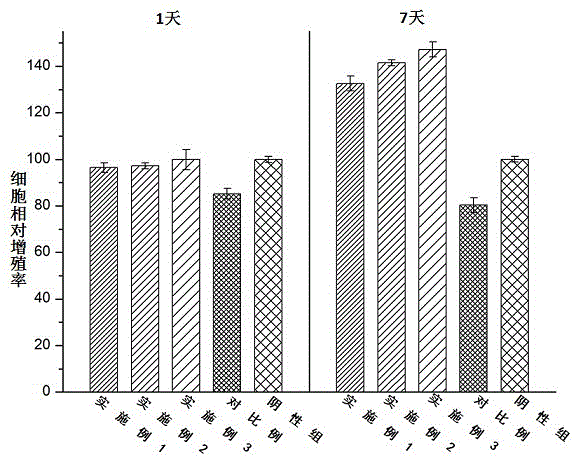
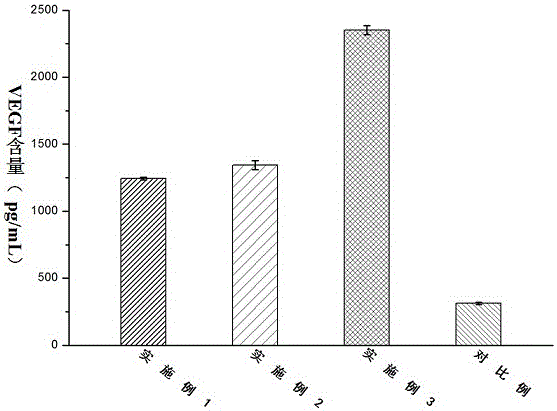
[0027] An antibacterial acellular dermal dressing capable of inducing vascularization is composed of acellular dermal matrix, polydopamine, vascular endothelial growth factor (VEGF) and antimicrobial peptide LL37; in parts by weight, it consists of 10 parts of acellular dermal matrix, polydopamine 0.25 parts, 0.01 parts of vascular endothelial growth factor, and 0.1 parts of antimicrobial peptide LL37; the vascular endothelial growth factor (VEGF), antimicrobial peptide LL37 were purchased from Thermo Fisher Scientifi Company of the United States.
Embodiment 2
[0029] An antibacterial acellular dermal dressing capable of inducing vascularization is composed of acellular dermal matrix, polydopamine, vascular endothelial growth factor (VEGF) and antimicrobial peptide LL37; in parts by weight, it consists of 10 parts of acellular dermal matrix, polydopamine 0.3 parts of vascular endothelial growth factor, 0.025 parts of vascular endothelial growth factor, and 0.3 parts of antibacterial peptide LL37; the vascular endothelial growth factor (VEGF) and antimicrobial peptide LL37 were purchased from Thermo Fisher Scientifi, USA.
Embodiment 3
[0031]An antibacterial acellular dermal dressing capable of inducing vascularization is composed of acellular dermal matrix, polydopamine, vascular endothelial growth factor (VEGF) and antimicrobial peptide LL37; in parts by weight, it consists of 10 parts of acellular dermal matrix, polydopamine 0.5 parts of vascular endothelial growth factor, 0.05 parts of vascular endothelial growth factor, and antimicrobial peptide LL370.5; the vascular endothelial growth factor (VEGF), antimicrobial peptide LL37 were purchased from Thermo Fisher Scientifi, USA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com