Method for preparing supercapacitor electrode material basic nickel-cobalt carbonate through hydrothermal method
A supercapacitor, nickel-cobalt carbonate technology, applied in hybrid capacitor electrodes, nickel carbonate, cobalt carbonate and other directions, can solve problems such as unsatisfactory electrochemical performance, and achieve improved capacity rate characteristics, improved electrochemical performance, and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
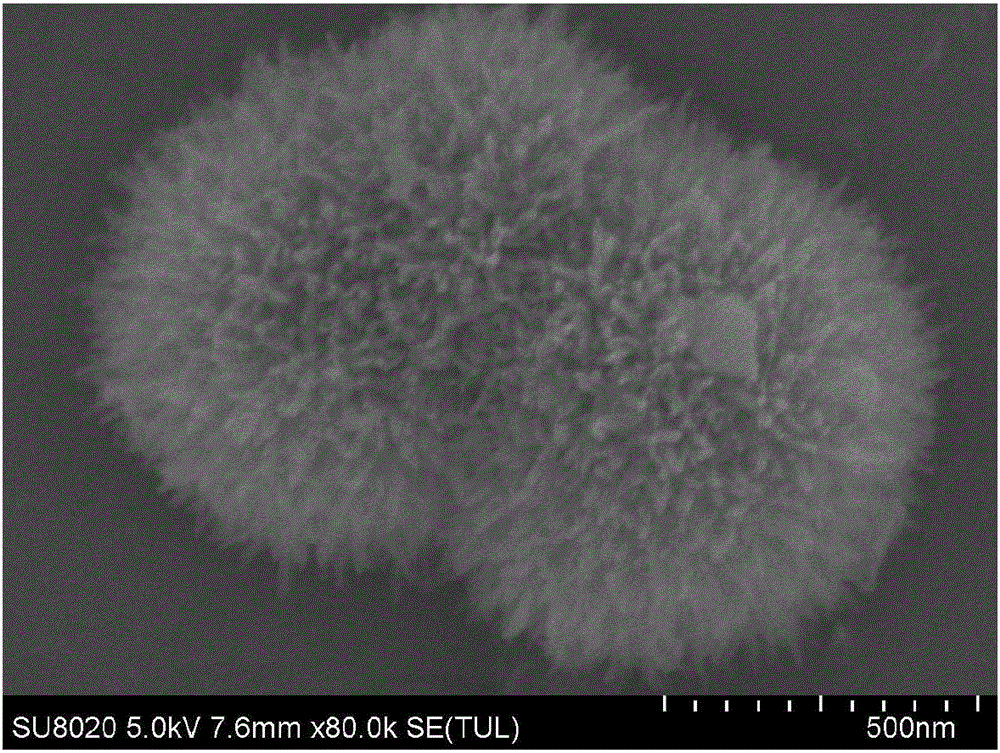
[0036] Weigh a certain amount of Ni(NO 3 ) 2 ·6H 2 O and urea were added to deionized water and stirred until the solids were completely dissolved, wherein: Ni(NO 3 ) 2 ·6H 2 The concentration of O is 0.05mol / L, and the concentration of urea is 0.125mol / L; pour the dissolved mixed solution into a high-pressure reactor, seal it, put it in an oven or a constant temperature box, and react at 90°C 14 hours, remove. After cooling to room temperature, the product was centrifuged, washed three times with deionized water, washed twice with absolute ethanol, and finally dried in vacuum at 80°C for 15 hours to obtain basic nickel carbonate (NiCH). Its surface morphology is as figure 1 As shown, it has a hairy needle-like aggregate structure, which is beneficial to the contact and wetting of the electrolyte, and makes full use of the surface area of the active material.
Embodiment 2
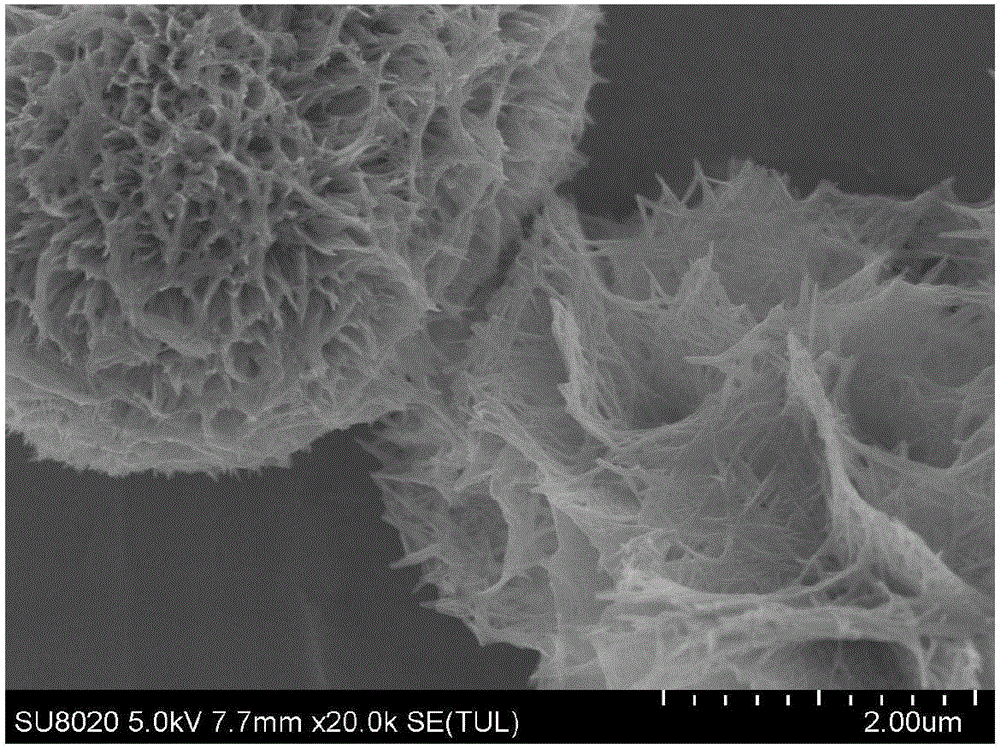
[0038]Weigh a certain amount of CoSO 4 ·7H 2 O and urea were added to deionized water and stirred until the solids were completely dissolved, in which: CoSO 4 ·7H 2 The concentration of O is 0.05mol / L, and the concentration of urea is 0.125mol / L; pour the dissolved mixed solution into a high-pressure reactor, seal it, put it in an oven or a constant temperature box, and react at 90°C 14 hours, remove. After cooling to room temperature, the product was centrifuged, washed three times with deionized water, washed twice with absolute ethanol, and finally vacuum-dried at 80°C for 15 hours to obtain basic cobalt carbonate (CoCH). Its surface morphology is as figure 2 shown.
Embodiment 3
[0040] Weigh a certain amount of Ni(NO 3 ) 2 ·6H 2 O. CoSO 4 ·7H 2 O and urea were added to deionized water and stirred until the solids were completely dissolved, wherein: Ni(NO 3 ) 2 ·6H 2 O and CoSO 4 ·7H 2 The total concentration of O is 0.13mol / L (the molar ratio of nickel salt and cobalt salt is 3:7), and the concentration of urea is 0.25mol / L; pour the dissolved mixed solution into the autoclave and seal it well. Put it into an oven or a constant temperature box, react at 90°C for 14 hours, and take it out. After cooling to room temperature, the product was centrifuged, washed three times with deionized water, washed twice with absolute ethanol, and finally vacuum-dried at 80°C for 15 hours to obtain basic nickel-cobalt carbonate (NiCoCH). Its surface morphology is as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com