Synthetic process for 2-hydrazinylpyridine derivative
A synthetic process, the technology of hydrazinopyridine, applied in the direction of organic chemistry, can solve the problems of low product yield, no safety protection, and many steps, so as to improve selectivity and reaction rate, ensure selectivity and efficiency, and react The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] (1) Synthesis of 2,3-dichloropyridine (using 2,3,6-trichloropyridine as raw material)
[0089] Add 500g of 2.3.6-trichloropyridine, 1800g of methanol, 20g of mixed catalyst (8%Pt / C:8%Pd / C=1:10) and 45g of pyridine into the reaction kettle, pass in hydrogen, and slowly drop 5 (weight)% sodium hydroxide methanol solution, maintain the pressure in the reactor to be 0.3Mpa, the temperature is 30 DEG C, after the hydrogenation is completed, replace the hydrogen, filter out the Pt / C and Pd / C catalysts for mechanical use, and receive the filtrate atmospheric pressure distillation Methanol is recovered, water is added to the remaining material, the temperature is lowered to crystallize, centrifuged and purified to obtain 2,3-dichloropyridine.
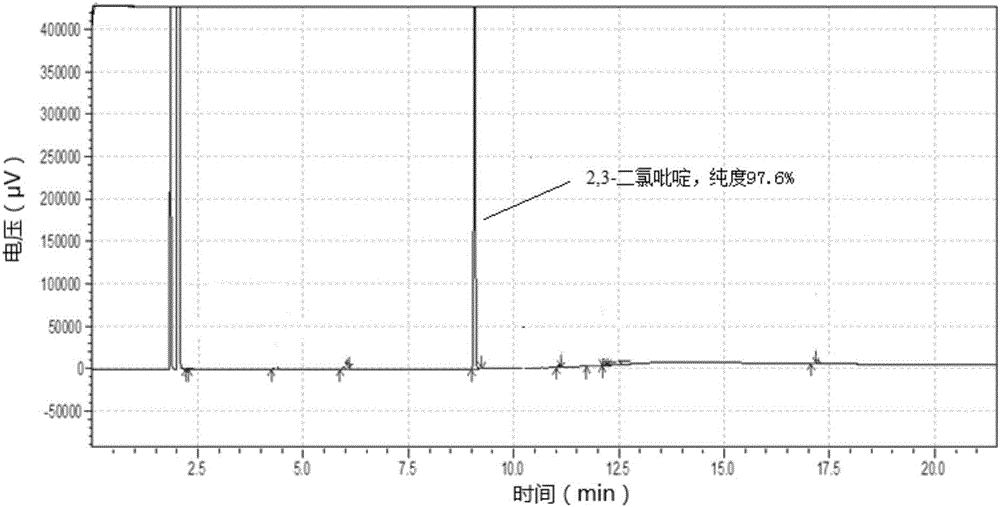
[0090] In step (1), the purity of 2,3-dichloropyridine is 97.6%, and the yield is 88.5%. After crystallization, the gas chromatogram without purification is as follows figure 1 shown.
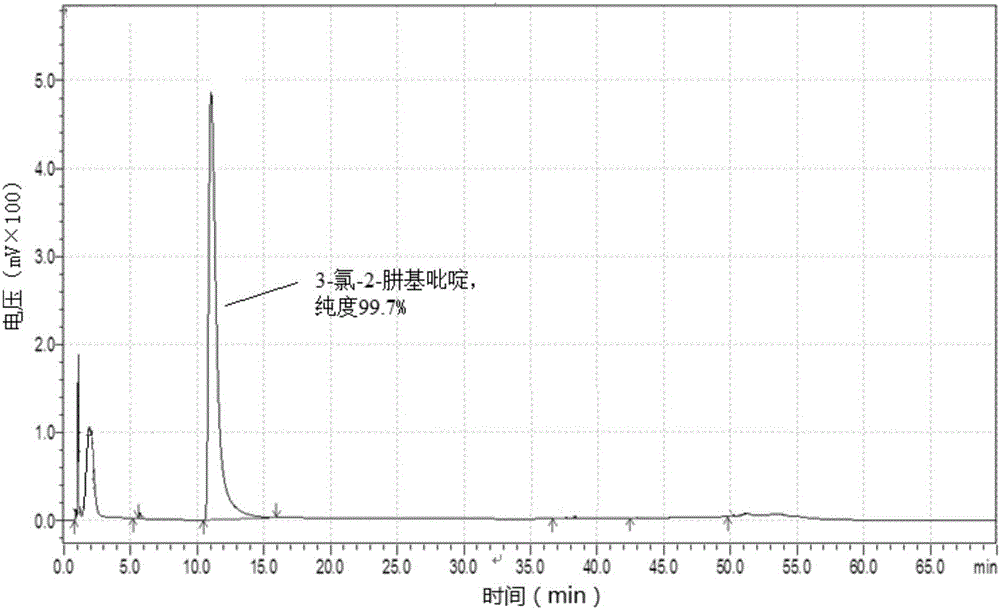
[0091] (2) Synthesis of 3-chloro-2 hydrazinopyrid...
Embodiment 2
[0095] (1) Synthesis of 2,3-dichloropyridine
[0096] 450g of 2.3.6-trichloropyridine, 1600g of ethanol, 20g of mixed catalyst (8%Pt / C:8%Pd / C=10:1) and 45g of triethylamine were added to the reactor, and hydrogen was introduced while slowly Add dropwise 5 (weight)% sodium hydroxide ethanol solution, keep the pressure in the reactor as 0.25Mpa, and the temperature is 25 to 30°C. After the hydrogenation is completed, replace the hydrogen, filter out the Pt / C and Pd / C catalysts, and receive The filtrate is distilled at normal pressure to recover methanol, water is added to the remaining material, the temperature is lowered to crystallize, centrifuged and purified to obtain 2,3-dichloropyridine.
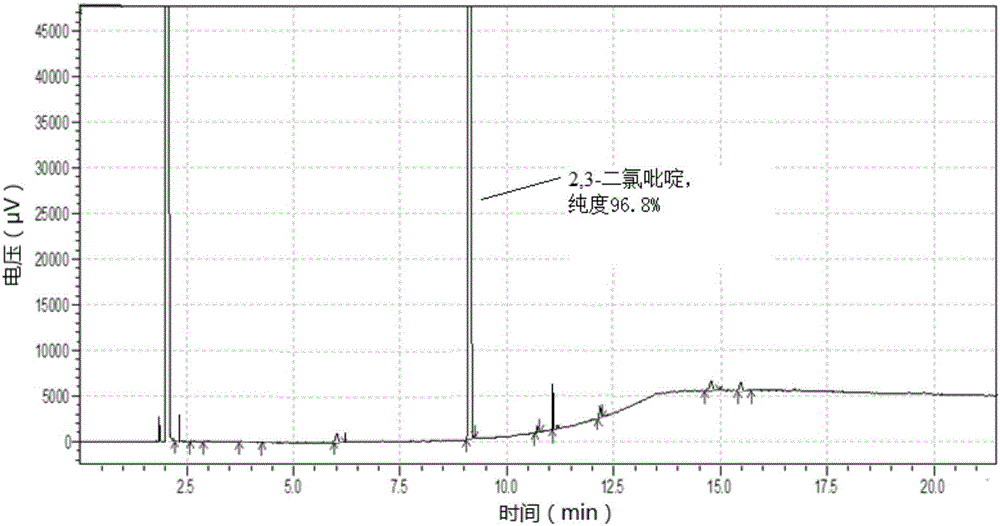
[0097] In step (1), the purity of 2,3-dichloropyridine is 96.8%, and the yield is 85%, and the gas chromatogram is as follows without purification after crystallization image 3 shown.
[0098] (2) Synthesis of 3-chloro-2 hydrazinopyridine
[0099] Mix 148g of 2,3-dichloropyridine and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com