Iron-based catalyst for preparing low-carbon olefin directly from synthesis gas and preparation method of iron-based catalyst
A technology of low-carbon olefins and catalysts, applied in the field of catalysts and their preparation, can solve problems such as easy overheating, easy deactivation of catalysts, and difficulty in removing heat from reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
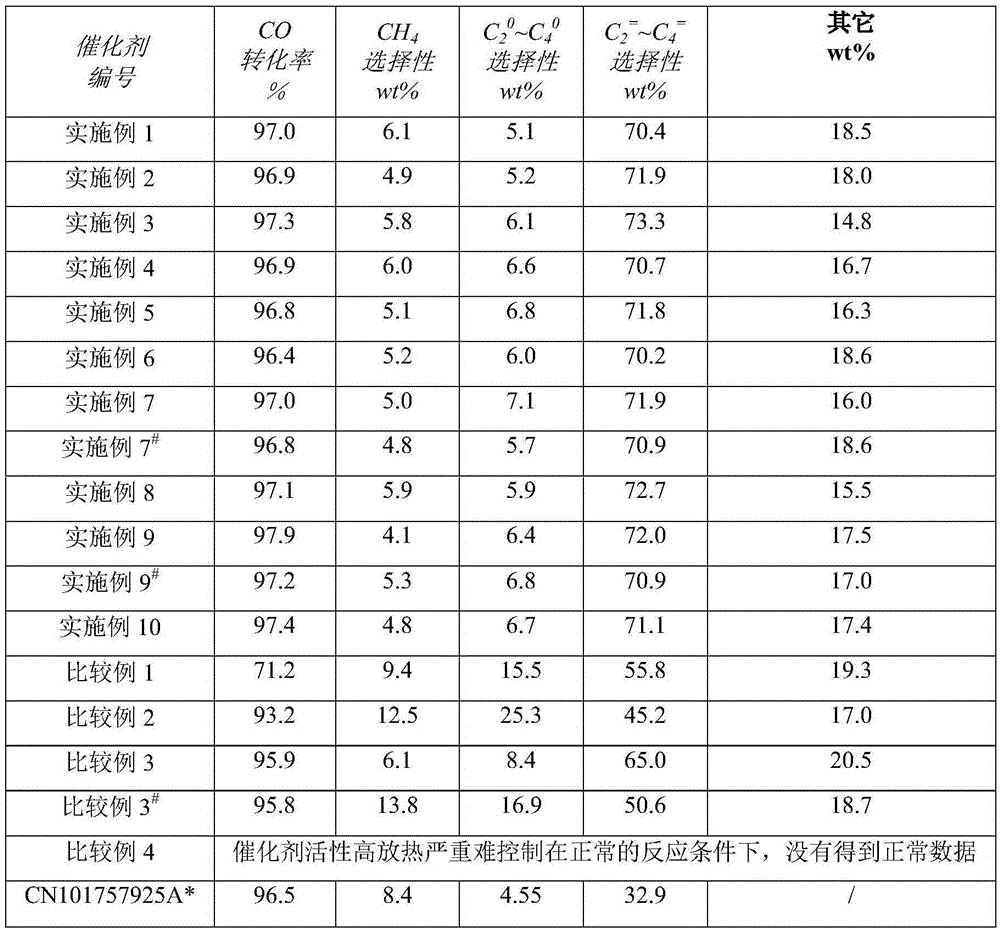
Examples
Embodiment 1
[0024] Get 40.0g of titanyl sulfate and dissolve it in 1000ml of water, make it into 0.25mol / L titanyl sulfate solution, the solution and 400g of 5 wt% ammonia water are co-precipitated and then centrifuged, washed with deionized water three times to obtain fresh The titanium oxide precipitate I;
[0025] Dissolve 606.03 g of ferric nitrate nonahydrate in a certain amount of water to prepare solution II;
[0026] Mix solution II and precipitate I, and peptize in a water bath at 40°C for 24 hours to obtain colloidal slurry III;
[0027] Dissolve 0.084g of potassium hydroxide in 1.00g of water to make potassium hydroxide solution
[0028] Cool the slurry III to 10 °C in a water bath at 10 °C, then add the above-mentioned potassium hydroxide to the cooled slurry III, and mix and beat at 10 °C;
[0029] Then add 0.098g of 15% by weight dilute sulfuric acid to the above slurry;
[0030] Finally, the pH value of the slurry was adjusted to 5 with ammonia water to obtain a uniform ...
Embodiment 2
[0035] Take 1072g of zirconium nitrate pentahydrate and dissolve it in 2500ml of water, make it into a 0.8mol / L zirconium nitrate solution, and the solution and 800g of 25% by weight ammonia water are co-precipitated and then centrifuged, and washed three times with deionized water to obtain fresh Zirconia Precipitation I;
[0036] Dissolve 606.03 g of ferric nitrate nonahydrate in a certain amount of water to prepare solution II;
[0037] Mix solution II and precipitate I, and peptize in a water bath at 100 °C for 0.5 h to obtain colloidal slurry III;
[0038]Dissolve 20.47g of cesium nitrate in 50.0g of water to prepare a cesium nitrate solution;
[0039] Add the above cesium nitrate solution to slurry III in a water bath at 100 °C, and mix and beat at 100 °C;
[0040] Then add 19.6g of 15% by weight dilute phosphoric acid to the above slurry;
[0041] Finally, the pH value of the slurry was adjusted to 1 with dilute nitric acid to obtain a uniform slurry IV (solid conten...
Embodiment 3
[0046] Get 80.0g of titanyl sulfate and dissolve it in 2000ml of water, make it into a 0.25mol / L titanyl sulfate solution, and this solution and 800g of 5 wt% ammonia water are co-precipitated and then centrifuged, and washed three times with deionized water to obtain fresh The titanium oxide precipitate I;
[0047] Dissolve 367.43g of ferric citrate in a certain amount of water to prepare solution II;
[0048] Mix solution II and precipitate I, and peptize in a water bath at 70°C for 12 hours to obtain colloidal slurry III;
[0049] Dissolve 4.8g sodium hydroxide in 10.00g water to make potassium hydroxide solution
[0050] Cool the slurry III to 40 °C in a water bath of 40 °C, then add the above-mentioned potassium hydroxide to the cooled slurry III, and mix and beat at 40 °C;
[0051] Then add 0.294g of 15% by weight dilute sulfuric acid to the above slurry;
[0052] Finally, the pH value of the slurry was adjusted to 3 with dilute ammonia water to obtain a uniform slurr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com