Dendritic cell-targeted pH-response type DNA vaccine delivery system and preparation method
A technology of DNA vaccines and dendritic cells, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulations, etc., which can solve DNA vaccine enzymatic hydrolysis, insufficient antigen presentation, and low transfection efficiency To achieve the effects of mild and controllable reaction conditions, good targeting of dendritic cells, and improved transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of DNA carrier
[0035] Dissolve 2 mg of DSPE-G2 in 100 μL of chloroform / methanol (v / v=4:1), inject 1 mL of rapidly stirring HEPES buffer (10 mM, pH 7.4), and continue stirring for 6 h to remove the organic solvent.
[0036] The particle size distribution and surface potential of DSPE-G2 nanoparticles were measured with a laser particle size analyzer. The results showed that the particle size of DSPE-G2 nanoparticles was 296.3±19.6nm, the dispersion coefficient was 0.269, and the surface potential was 31.5±6.1mV.
Embodiment 2
[0037] Example 2: Preparation of binary complexes
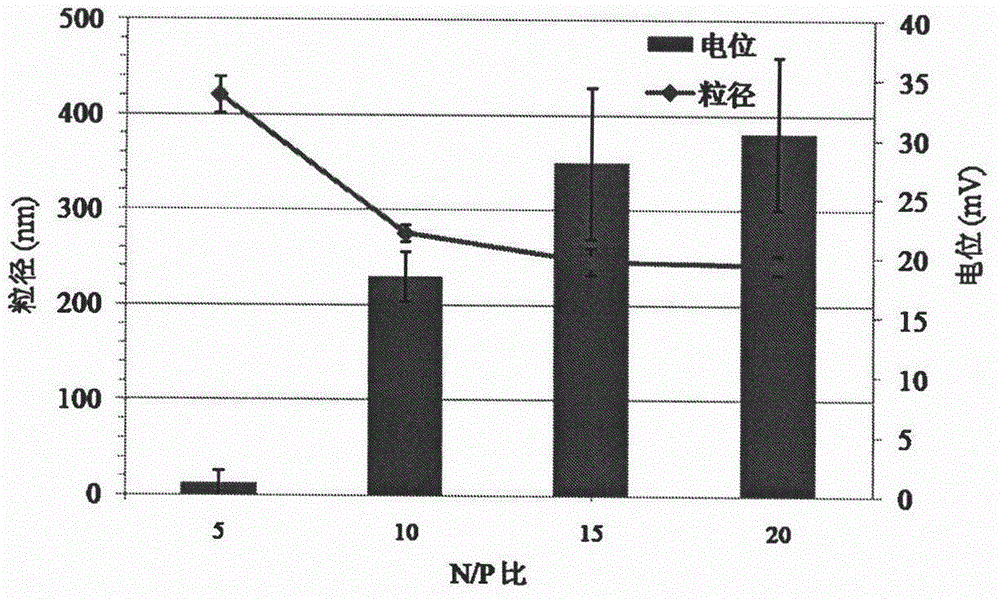
[0038] The pDNA was dissolved in HEPES buffer (10 mM, pH 7.4) to adjust the concentration to 0.2 mg / mL. The concentration of DSPE-G2 solution was adjusted to 0.1, 0.2, 0.4, 0.5, 0.6, 1, 1.5, 2 mg / mL with HEPES buffer (10 mM, pH 7.4). Mix different concentrations of DSPE-G2 solution and pDNA solution in equal volumes, corresponding to N / P ratios of 1, 2, 4, 5, 6, 10, 15, and 20, vortex for 1 min, and incubate at room temperature for 20 min.
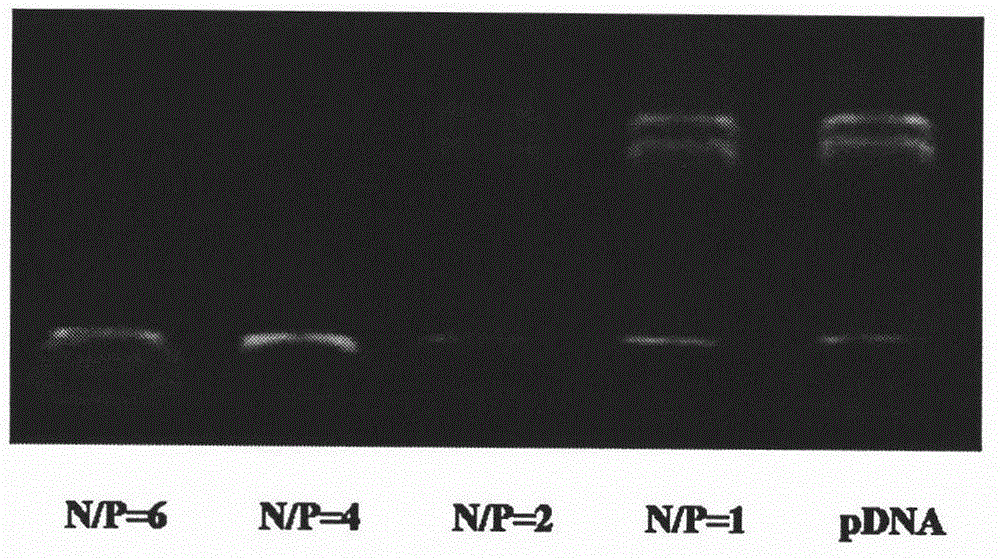
[0039] Gel retardation experiments were performed on binary complexes to test the ability of DSPE-G2 to compress pDNA. Take 10 μL of the binary complex solution and mix it with the loading buffer, add it to the loading hole of 1% agarose gel containing Goldview nucleic acid dye, immerse in the TAE electrophoresis buffer, and electrophoresis at a constant voltage of 90V for 30 min. Gel retardation bands were observed under UV light. The results show that when the N / P ratio is ≥ 4, DSPE-...
Embodiment 3
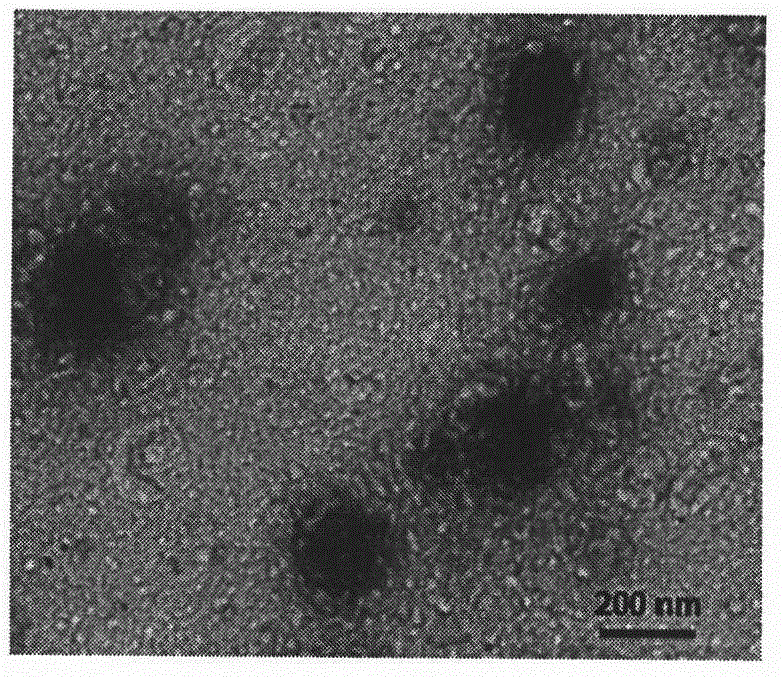
[0041] Example 3: Preparation method of a dendritic cell-targeted pH-responsive DNA vaccine delivery system
[0042] Mix 50 μL of 0.2 mg / mL pDNA solution with 50 μL of 2 mg / mL DSPE-G2 solution, vortex for 1 min, and incubate at room temperature for 20 min. Take 100 μL of 1 mg / mL PAH-Cit solution and mix it with the binary complex solution, and incubate at room temperature for 30 min. Add 200 μL of ternary complex solution dropwise to 300 μL of 0.5 mg / mL MCS solution under stirring, and keep stirring for 30 min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com