A kind of manganese-based catalyst for the treatment of volatile organic compounds and its preparation and application
A technology of volatile organic compounds and manganese-based catalysts, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, heterogeneous catalyst chemical elements, etc., can solve the problem that the activity is not as good as precious metals, etc. Achieve high stability and water resistance, long life and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
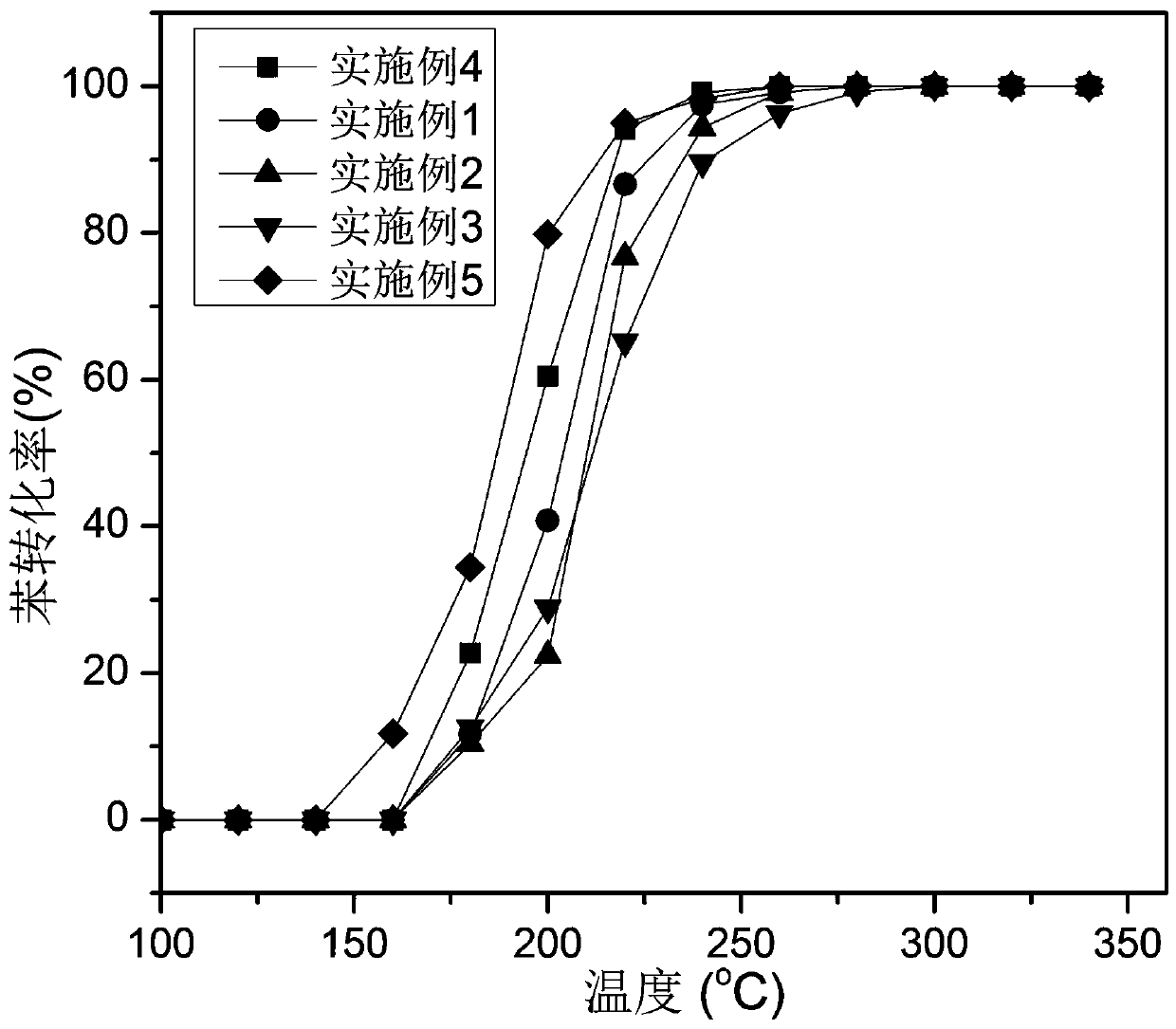
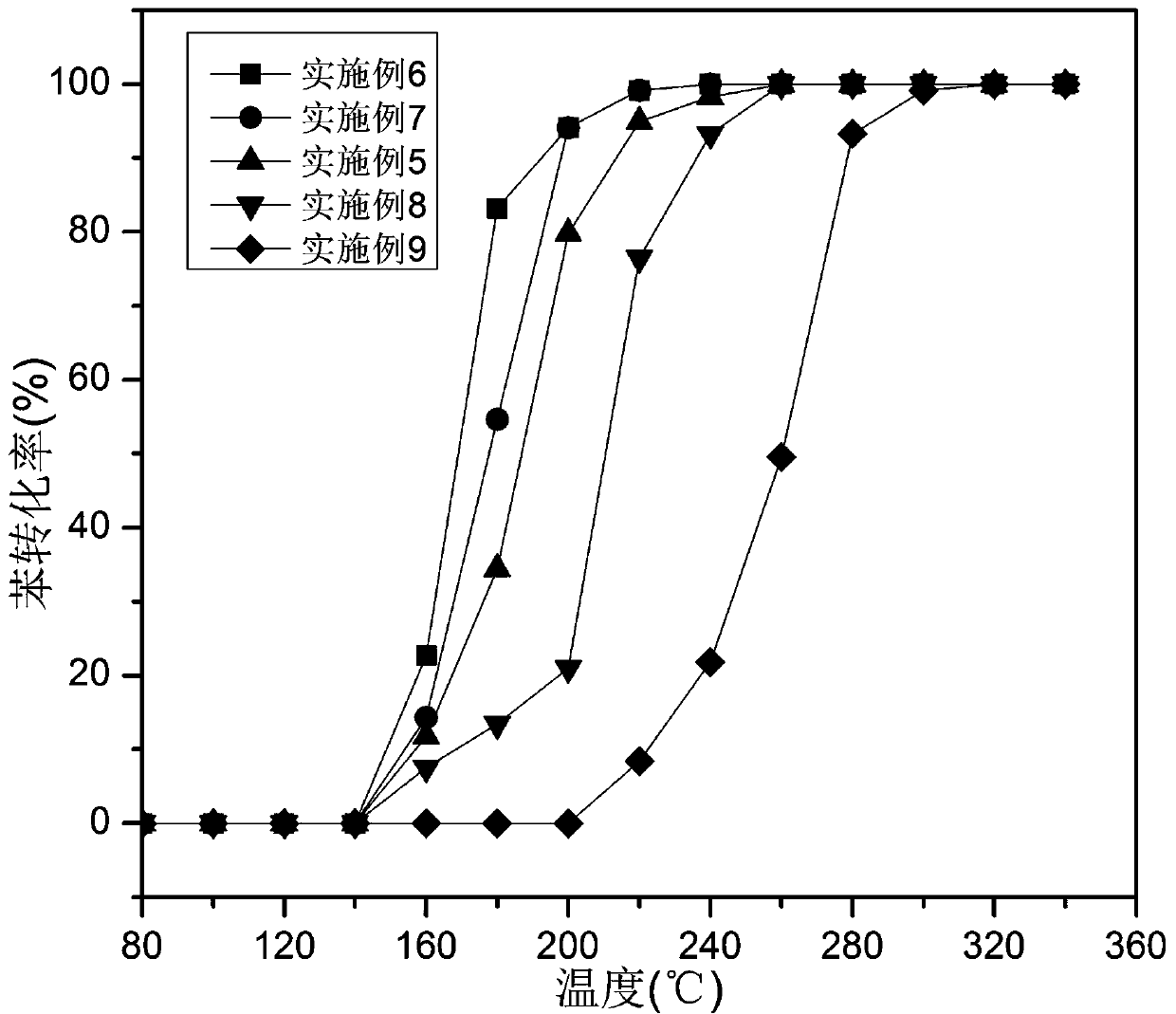
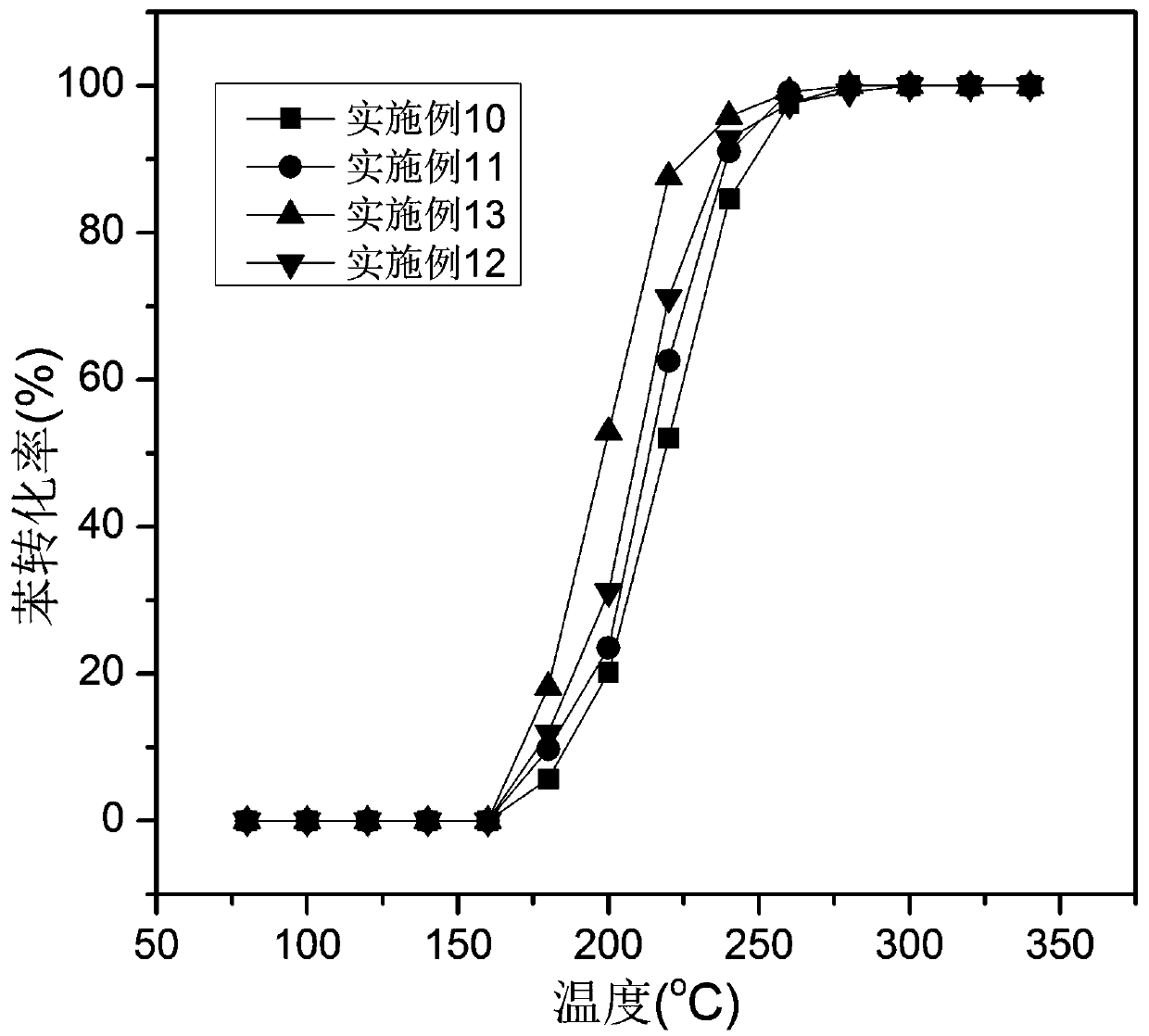
Examples
preparation example Construction
[0039] The following is a detailed description of the preparation of the manganese-based catalyst in the present invention, that is, the oxalate colloidal co-precipitation method.
[0040] The concrete operation of oxalate colloidal coprecipitation method is as follows:
[0041] Manganese salts and other metal salts are dissolved in absolute ethanol and stirred at room temperature to prepare a uniform metal solution. Other metal salt solutions include one or two of cobalt salts, cerium salts, and chromium salts. Oxalic acid is dissolved in absolute ethanol to form a uniform and transparent oxalic acid solution at room temperature. Slowly drop the oxalic acid solution into the metal solution, continue to stir for 4-6 h, and alcoholyze the metal salt. The obtained metal solid solution solution is vacuum filtered to obtain a solid material, and then dried in a blast oven at 60-80° C. to obtain a solid layer. The solid layer is calcined in a muffle furnace at 300-700 DEG C to o...
Embodiment 1
[0048] Embodiment 1: manganese-cobalt composite oxide
[0049] Weigh 1.07 g of manganese nitrate aqueous solution with a mass fraction of 50% and 5.24 g of cobalt nitrate hexahydrate and dissolve them in 200 mL of absolute ethanol, stir until dissolved, and form a uniform and transparent metal solution. Another 3.62 g of oxalic acid dihydrate was weighed and dissolved in 50 mL of absolute ethanol, and stirred until dissolved to form a uniform and transparent oxalic acid solution. Slowly drop the oxalic acid solution into the metal solution, and continue stirring for 4 h to form a solid suspension after all the solution is dissolved. The suspension was vacuum-filtered, dried in a blast oven at 70 °C for 12 h, and then roasted in a muffle furnace. The temperature was raised to 500 °C at a heating rate of 2 °C / min and kept for 4 h to obtain Mn 1 co 3 o x catalyst.
[0050] mn 1 co 3 Ox catalyst performance evaluation to benzene catalytic combustion, operation is as follows:...
Embodiment 2
[0054] Example 2: Manganese-cobalt composite oxide
[0055] Weigh 0.73 g of manganese nitrate aqueous solution with a mass fraction of 50% and 5.82 g of cobalt nitrate hexahydrate and dissolve them in 200 mL of absolute ethanol, stir until dissolved, and form a uniform and transparent metal solution. Another 3.62 g of oxalic acid dihydrate was weighed and dissolved in 50 mL of absolute ethanol, and stirred until dissolved to form a uniform and transparent oxalic acid solution. Slowly drop the oxalic acid solution into the metal solution, and continue stirring for 4 h to form a solid suspension after all the solution is dissolved. The suspension was vacuum-filtered, dried in a blast oven at 70 °C for 12 h, and then roasted in a muffle furnace. The temperature was raised to 500 °C at a heating rate of 2 °C / min and kept for 4 h to obtain Mn 1 co 5 o x catalyst.
[0056] mn 1 co 5 Ox catalyst performance evaluation to benzene catalytic combustion, operation is as follows:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com