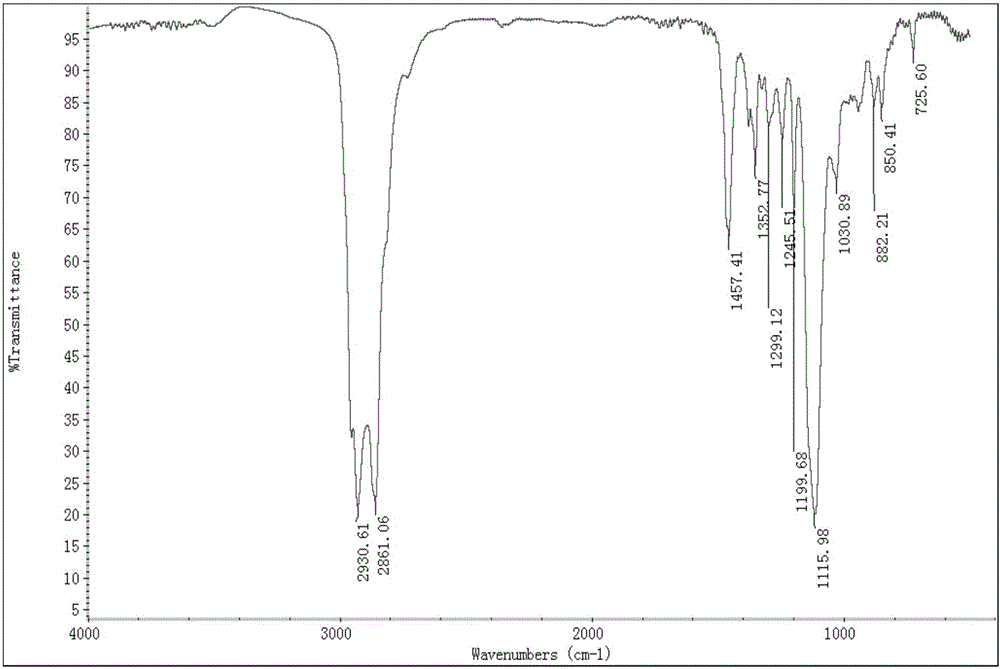
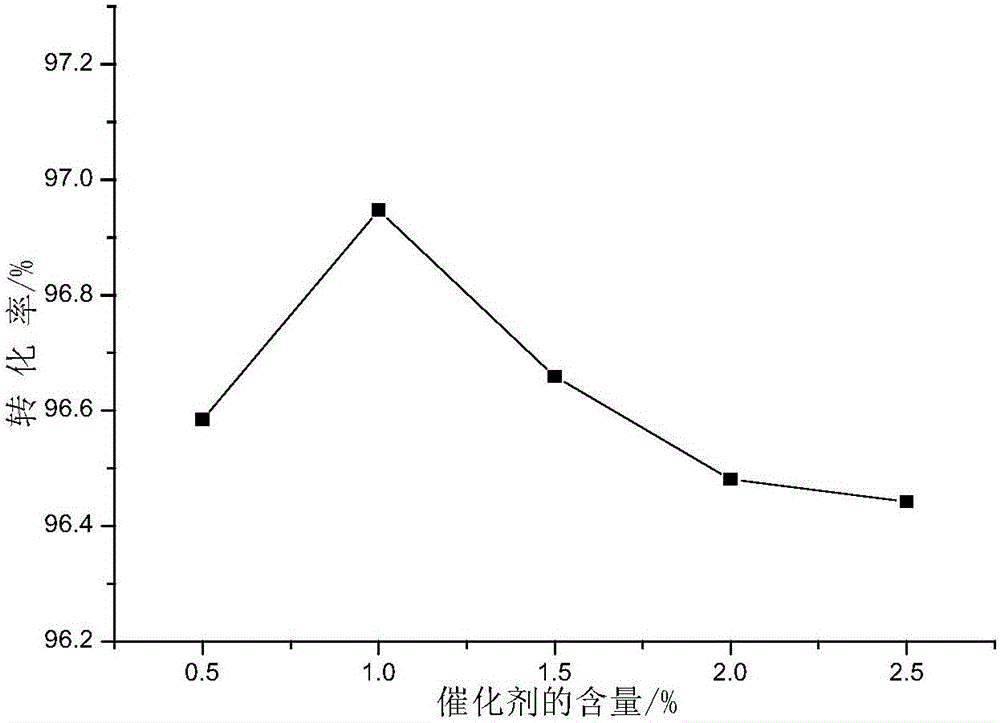
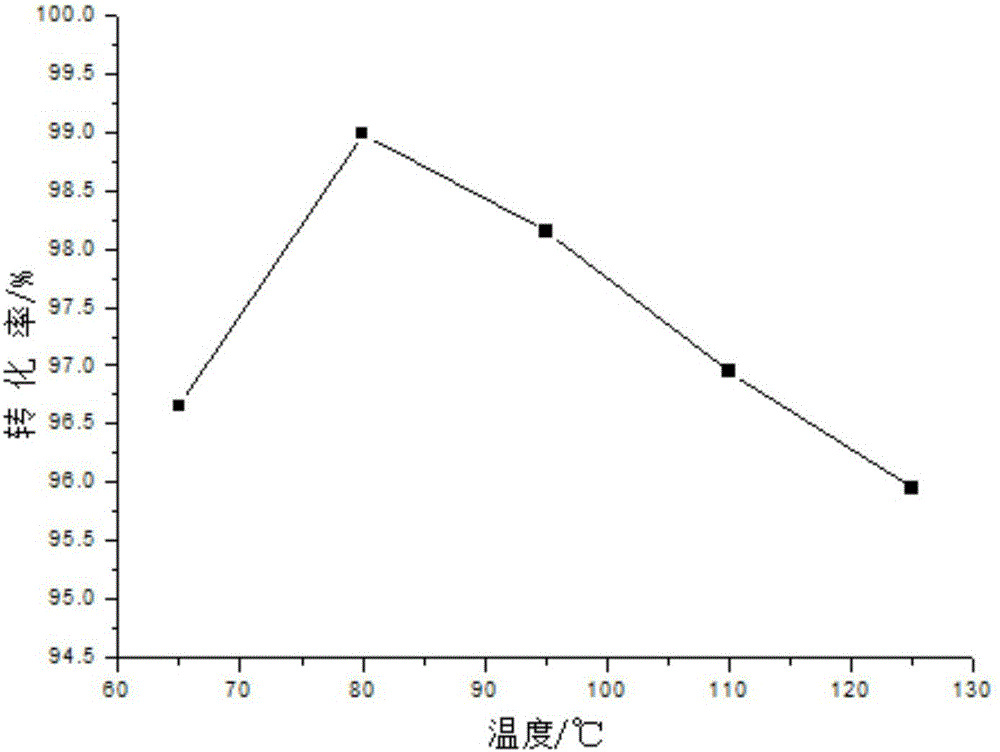
Method for synthesizing diethylene glycol methyl hexyl ether
A technology of diethylene glycol and methylhexyl ether, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of ether, etc. It can solve the problems of difficult to realize large-scale production, difficult control of sodium alkoxide reaction, low conversion rate of sodium alkoxide, etc. problems, to achieve the effect of low toxicity, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment one: a kind of method for synthesizing diethylene glycol methylhexyl ether provided by the present invention comprises the following steps, (1) raw material sodium hydroxide is placed in the reactor, then dilute hydrochloric acid solution is slowly added dropwise into the reactor In the reaction, the reaction generates sodium chloride, and the chemical reaction equation is:
[0024] NaOH+HCl→NaCl+H 2 O;
[0025] (2) Add diethylene glycol monohexyl ether dropwise to the reaction kettle in step (1), heat the reaction kettle to 80° C., and continuously feed hydrogen into the reaction kettle, and connect the reaction kettle to a reflux device to recover the reaction kettle Hydrochloric acid, the sodium alkoxide intermediate product that obtains is passed in the next stage reactor, and described chemical reaction equation is:
[0026] C 6 h 13 (OCH 2 CH 2 ) 2 OH+NaCl→C 6 h 13 (OCH 2 CH 2 ) 2 ONa+HCl;
Embodiment 2
[0038] Embodiment two: the method for synthesizing diethylene glycol methylhexyl ether disclosed by the present invention comprises the following steps, (1) putting the raw material sodium hydroxide in the reactor, then slowly adding dilute hydrochloric acid solution dropwise into the reactor for reaction The mass ratio of sodium hydroxide and hydrochloric acid is 1:0.5 to react to generate sodium chloride, and the end point of the reaction can be controlled by a pH indicator. The sufficient time for this reaction is 2h, and the chemical reaction equation is:
[0039] NaOH+HCl→NaCl+H 2 O;
[0040] (2) Add diethylene glycol monohexyl ether dropwise to the reactor in step (1), heat the reactor to 120°C, and continuously feed hydrogen into the reactor, the flow rate of hydrogen is 3ml / min, diethylene glycol The mass ratio of glycol monohexyl ether to sodium chloride is 1:1.5, and the reaction kettle is connected with the reflux device to recover the hydrochloric acid generated b...
Embodiment 3
[0052] Embodiment three: the method for synthesizing diethylene glycol methylhexyl ether disclosed by the present invention comprises the following steps, (1) putting the raw material sodium hydroxide in the reactor, then slowly adding dilute hydrochloric acid solution dropwise into the reactor for reaction , the mass ratio of sodium hydroxide and hydrochloric acid is 1:1 and reacts to generate sodium chloride, and the reaction terminal can be controlled by a pH indicator. The sufficient time for this reaction is 1.5h, and the chemical reaction equation is:
[0053] NaOH+HCl→NaCl+H 2 O;
[0054] (2) Add diethylene glycol monohexyl ether dropwise to the reactor in step (1), heat the reactor to 100°C, and continuously feed hydrogen into the reactor, the flow rate of hydrogen is 5ml / min, diethyl ether The mass ratio of glycol monohexyl ether to sodium chloride is 1:2, the reaction kettle is connected with the reflux device to recover the hydrochloric acid generated by the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com