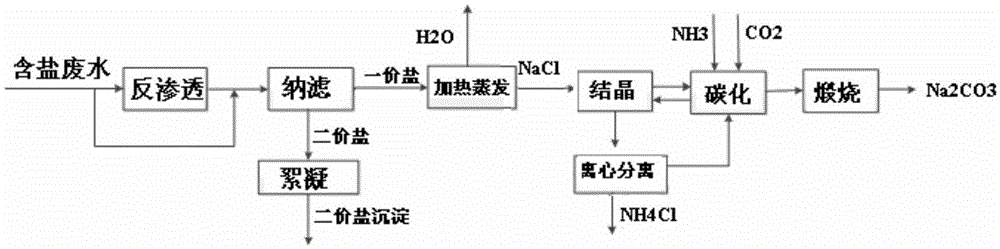
Method for co-producing sodium carbonate and ammonium chloride by utilizing salt-containing wastewater
A technology for saline wastewater and ammonium chloride, applied in the direction of ammonium chloride, ammonium halide, carbonate preparations, etc., to achieve the effect of solving the problem of disposal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
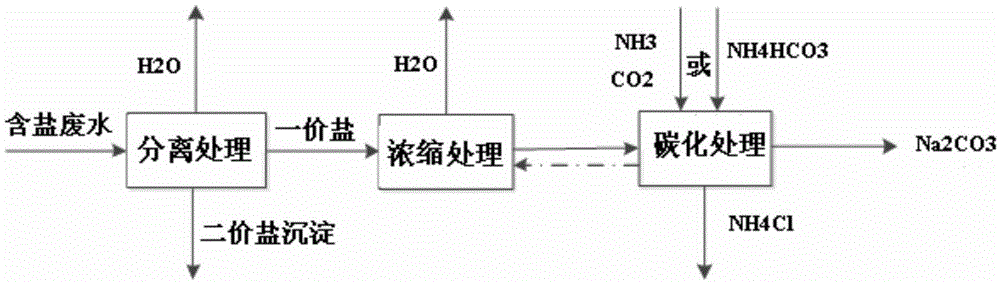
[0041] This example is used to illustrate the use of figure 2 The method shown is a method for co-producing soda ash and ammonium chloride by using salty wastewater.
[0042] Synthetic saline wastewater was used as raw water to simulate wastewater discharged from coal chemical enterprises, and its water quality parameters are shown in Table 1.
[0043] (1) Set the flow to 10m 3 The above-mentioned synthetic salt-containing wastewater of / h was subjected to reverse osmosis treatment, and the flow rate was 6.6 m 3 Product water and flow rate of 3.4m / h 3 The reverse osmosis concentrated water with a NaCl concentration of 100g / L per hour, and the product water is returned to the production system for recycling. The conditions of the reverse osmosis treatment include: the pressure is 5MPa, the temperature is 30°C, the membrane pore size is 0.5nm, and the membrane material is for cellulose acetate.
[0044] (2) Set the flow to 3.4m 3 / h of reverse osmosis concentrated water is...
Embodiment 2
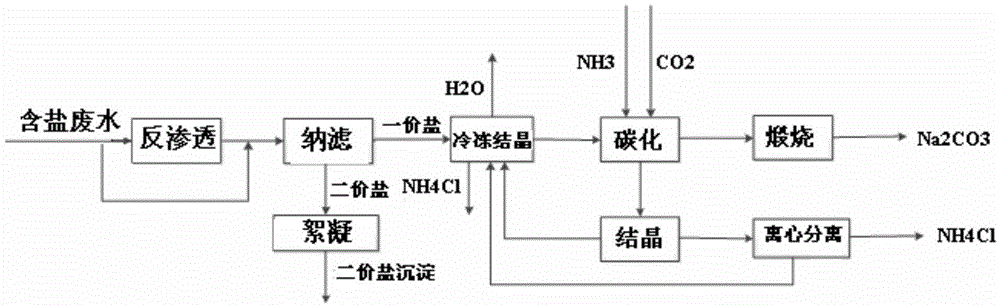
[0057] This example is used to illustrate the use of image 3 The method shown is a method for co-producing soda ash and ammonium chloride by using salty wastewater.
[0058] (1) Set the flow to 11m 3 The synthetic salt-containing wastewater described in Table 1 of / h is subjected to reverse osmosis treatment, and the obtained flow rate is 7m 3 4m of product water and flow per hour 3 The reverse osmosis concentrated water with a NaCl concentration of 110g / L per hour, and the product water is returned to the production system for recycling. The conditions of the reverse osmosis treatment include: the pressure is 5.2MPa, the temperature is 28°C, the membrane pore size is 0.5nm, and the membrane The material is cellulose acetate.
[0059] (2) Set the flow to 4m 3 / h reverse osmosis concentrated water is treated by nanofiltration, and the flow rate is 1.1m 3 / h of divalent salt-rich brine and flow rate of 2.9m 3 The NaCl concentration of / h is 105g / L of monovalent salt-rich ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Membrane pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com