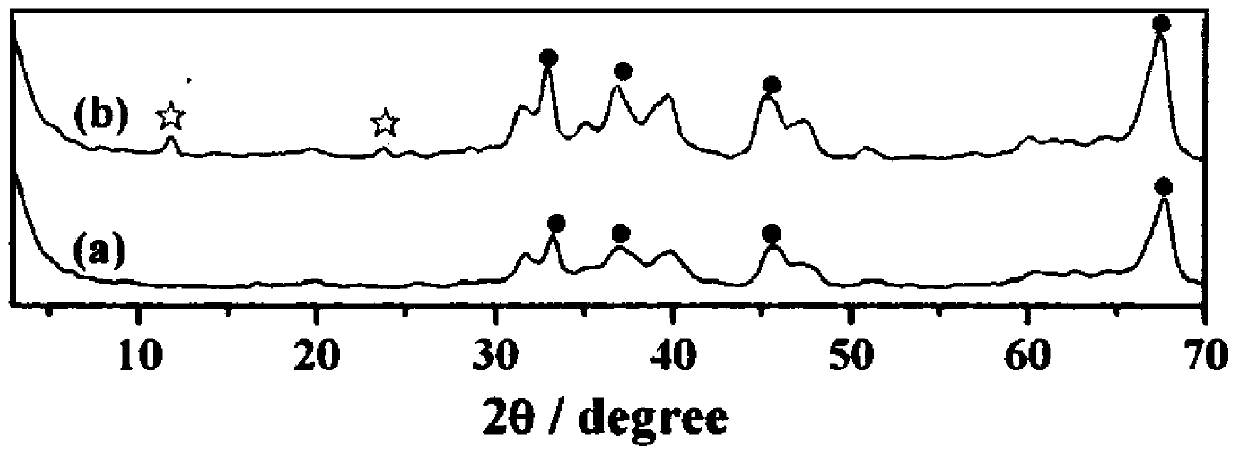
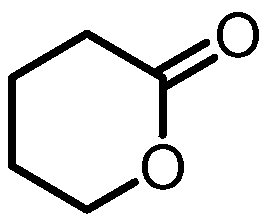
A kind of dehydrogenation catalyst and the method for manufacturing delta-valerolactone
A dehydrogenation catalyst, copper-based catalyst technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve the problem of short service life of catalysts, difficult product separation, The catalyst is easy to sinter and other problems, so as to improve the selectivity, simplify the product separation process, and achieve the effect of good physical structure and performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Catalyst preparation:
[0043] Weigh 102g of Al 2 o 3 (1mol), mix it with 100ml of aqueous urea (CO(NH 2 ) 2 : 0.005mol) mixed evenly, placed in a 2L autogenous autoclave, kept at 90°C for 24h, then added 700ml of Mg(NO 3 ) 2 (0.02mol) aqueous solution, and heated up to 130°C, kept for 24h, washed with deionized water, dried at 120°C, and then roasted at 450°C for 8h to obtain LDHs-modified alumina composite MaterialLDHs-Al 2 o 3 .
[0044] Prepare a certain concentration of Cu(NO 3 ) 2 (where Cu(NO 3 ) 2 The amount of substance is an aqueous solution of 0.085mol), and then 100g of the LDHs-Al prepared above 2 o 3 into Cu(NO 3 ) 2 After fully shaken in the aqueous solution, place it in a water bath constant temperature shaker for 24 hours, keep the temperature of the water bath at about 70°C during this period, and shake at a rate of 130-140r / min. The obtained sample was washed 3-5 times with deionized water at room temperature, dried at 120°C, and then ...
Embodiment 2
[0051] Catalyst preparation:
[0052] With embodiment 1, difference is that alkaline substance CO(NH 2 ) 2 The dosage is 0.014mol, Mg(NO 3 ) 2 The dosage is 0.08mol, Cu(NO 3 ) 2 The dosage is 0.42mol, the prepared catalyst precursor is 131.2g, and the estimated active component copper loading is 23.7%.
[0053] Preparation of δ-valerolactone:
[0054] Same as in Example 1, the difference is that the carrier gas is replaced by hydrogen, and the reaction conditions are replaced by an absolute pressure of 0.009 MPa and 240°C. Intermittent sampling is carried out GC analysis to reaction liquid, can find out that after reaction carries out 20h, reaction enters steady state, and wherein reaction conversion rate reaches 99.6%, δ-valerolactone selectivity reaches 96.5%, and n-valeric acid is not found in by-product. After the device has been continuously operated for 1000 hours, there is no significant difference between the composition of the reaction liquid and the compositio...
Embodiment 3
[0056] Catalyst preparation:
[0057] With embodiment 1, difference is that alkaline substance CO(NH 2 ) 2 The dosage is 0.01mol, Mg(NO 3 ) 2 The dosage is 0.05mol, Cu(NO 3 ) 2 The dosage is 0.22mol, the prepared catalyst precursor is 116.1g, and the estimated active component copper loading is 13.9%.
[0058] Preparation of δ-valerolactone:
[0059] Same as in Example 1, the difference is that the carrier gas is replaced by nitrogen, and the reaction conditions are replaced by an absolute pressure of 0.005 MPa and 220°C. Intermittent sampling was carried out on the reaction liquid for GC analysis. It can be seen that after the reaction was carried out for 18 hours, the reaction entered a steady state, wherein the reaction conversion rate reached 99.9%, and the δ-valerolactone selectivity reached 98.5%. After the device has been continuously operated for 500 hours, there is no significant difference between the composition of the reaction liquid and the composition afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com