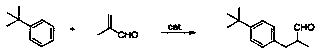A kind of preparation method of lilial
A technology of lily of the valley aldehyde and methacrolein, applied in the field of preparation of lily of the valley aldehyde, can solve the problems of affecting the quality, quality and application effect of lily of the valley aldehyde, insufficient reaction of reactants, and many by-products of reaction products, etc. High yield, sufficient reaction, and the effect of inhibiting polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1) In a 2L reaction flask, add tert-butylbenzene (805g, 6mol) and dichloromethane (510g, 6mol), place it in a low-temperature reaction bath at -20°C, and add tin tetrachloride (261g, 1mol) and sulfonic acid resin (amberlyst15) 10g, stirring and mixing for 5min;
[0059] 2) At -20°C, add 2-methacrolein (140g, 2mol) drop by drop, after 2.5h the dropwise addition is completed, and continue to keep warm for 1.5h;
[0060] 3) Add 400g of 2N hydrochloric acid to quench the reaction, wash with water 3 times, each time with 10mL of water; dry the organic phase with anhydrous MgSO4, moisture ≤ 1.0%. Distill the organic phase at 25°C under reduced pressure of 350mmHg to remove dichloromethane; obtain the crude lyral; carry out rectification on the crude product, collect 89°C / 1mmHg fraction, the fraction quality is 286g, GC content ≥ 98%, water content < 0.3 %, the lyral productive rate is 73%; the methylene chloride recovery rate is 96%, and the tert-butylbenzene recovery rate i...
Embodiment 2
[0062] 1) In a 2L reaction flask, add tert-butylbenzene (805g, 6mol) and 1,2-dichloroethane (390mL, 6mol), place it in a low-temperature reaction bath at -10°C, and add titanium tetrachloride (190g, 1mol) and sulfonic acid resin (amberlyst15) 10g, stir and mix for 30min;
[0063] 2) At -10°C, add 2-methacrolein (140g, 2mol) drop by drop, after 2.5h the dropwise addition is completed, and continue to keep warm for 3h;
[0064] 3) Add 200g of 2N sulfuric acid to quench the reaction, wash three times with 10mL each time; dry the organic phase with anhydrous MgSO4, the moisture content is ≤1.0%. The organic phase was evaporated at 35°C and 350mmHg under reduced pressure to remove 1,2-dichloroethane; the crude lyral was obtained; the crude product was rectified, and the 89°C / 1mmHg fraction was collected. The fraction quality was 204g, and the GC content was ≥98%. The water content is less than 0.3%, and the yield of lilial is 50%; the recovery rate of 1,2-dichloroethane is 98%, an...
Embodiment 3
[0066] 1) In a 2L reaction flask, add tert-butylbenzene (805g, 6mol) and chloroform (390mL, 6mol), place it in a low-temperature reaction bath at -10°C, add titanium tetrachloride (190g, 1mol) and formaldehyde Sulfuric acid (38.4g, 0.4mol), stirred and mixed for 10min;
[0067] 2) At -10°C, add 2-methacrolein (140g, 2mol) drop by drop, after 2.5h the dropwise addition is completed, and continue to keep warm for 3h;
[0068] 3) Add 200g of 2N sulfuric acid to quench the reaction, wash three times with 10mL each time; dry the organic phase with anhydrous MgSO4, the moisture content is ≤1.0%. The organic phase was at 38°C, and the chloroform was evaporated under reduced pressure at 350mmHg; the crude lyral was obtained; the crude product was rectified, and the fraction at 89°C / 1mmHg was collected. The yield of lanaldehyde is 67%; the recovery rate of chloroform is 98%, and the recovery rate of tert-butylbenzene is 97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com