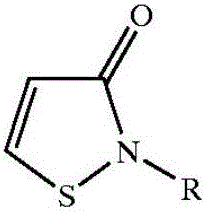
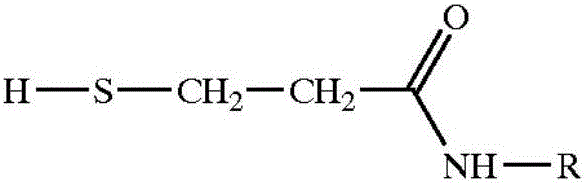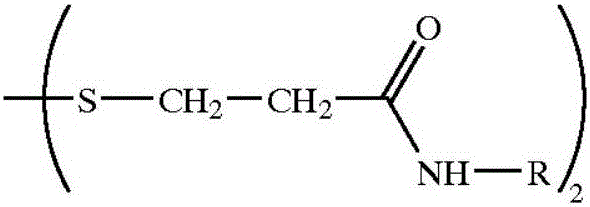Preparation method of high-purity 2-alkyl-4-isothiazoline-3-ketone
An isothiazoline, high-purity technology is applied in the field of preparation of high-purity 2-alkyl-4-isothiazolin-3-ones, which can solve the problem of low recovery rate, high volatility of halogenated hydrocarbon solvents, and increase of industrial production. Cost and other issues, to achieve the effect of extending the shelf life and service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]Suspend 500 kilograms of dithiodipropionamide in the above general formula (III) in 2000 kilograms of ethyl acetate, control the temperature at 15-25 degrees centigrade with cooling brine, and pass through the production of isothiazolinone. Hydrogen chloride tail gas, the tail gas theoretically releases 36 kg, and the molar ratio of the obtained amide to hydrogen chloride is 1:0.62. After the introduction of hydrogen chloride is completed, the reaction temperature in the kettle is controlled at 19-25 degrees Celsius under stirring, and 600 kg of chlorine gas is evenly introduced , to obtain the hydrochloride salt of 2-methyl 4-isothiazolin-3-one, filter to obtain solid hydrochloride, wash the hydrochloride with new ethyl acetate, dissolve it in 400 kg of water, and wash it with sodium carbonate And until the pH is 5-7, after high-pressure liquid chromatography analysis, the content of 2-methyl-4-isothiazolin-3-one obtained is 40%, 5-chloro-2-methyl-4-isothiazoline The -3...
Embodiment 2
[0040] According to Embodiment 1, the order of addition is adjusted as follows, after hydrogen chloride is passed into 2000 kg of ethyl acetate, then 500 kg of amide is added to stir, and then 600 kg of chlorine gas is passed into, the obtained hydrochloride is dissolved in 400 kg of water, and Sodium carbonate is neutralized until the pH value of the solution is 5-7, and the content of 2-methyl-4-isothiazolin-3-one obtained after high-pressure liquid chromatography analysis is 39%, 5-chloro-2-methyl-4 - The isothiazolin-3-one content is 0.16%. The yield of 2-methyl-4-isothiazolin-3-one was 72%.
[0041] Above-mentioned embodiment illustrates, first passes into hydrogen chloride and then adds amide in solvent, than the 2-methyl-4-isothiazolin-3-ketone purity in the final product obtained by amide absorption is slightly lower, but both can obtain relatively High yield and high purity 2-methyl-4-isothiazolin-3-one product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com