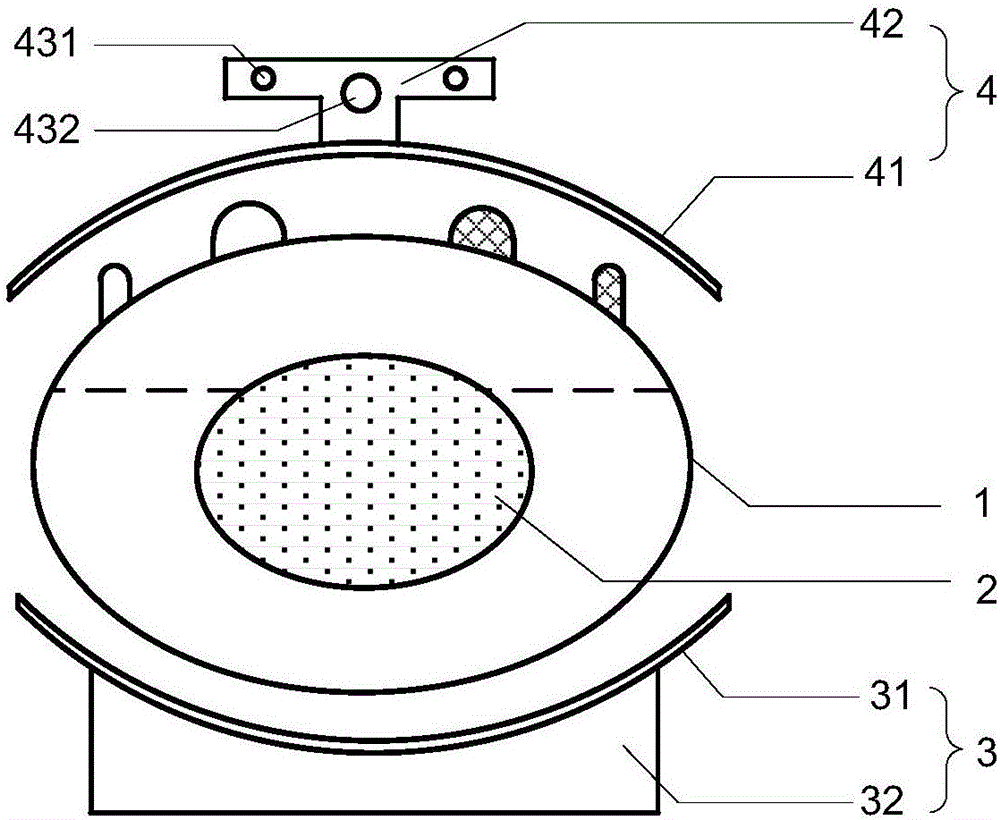
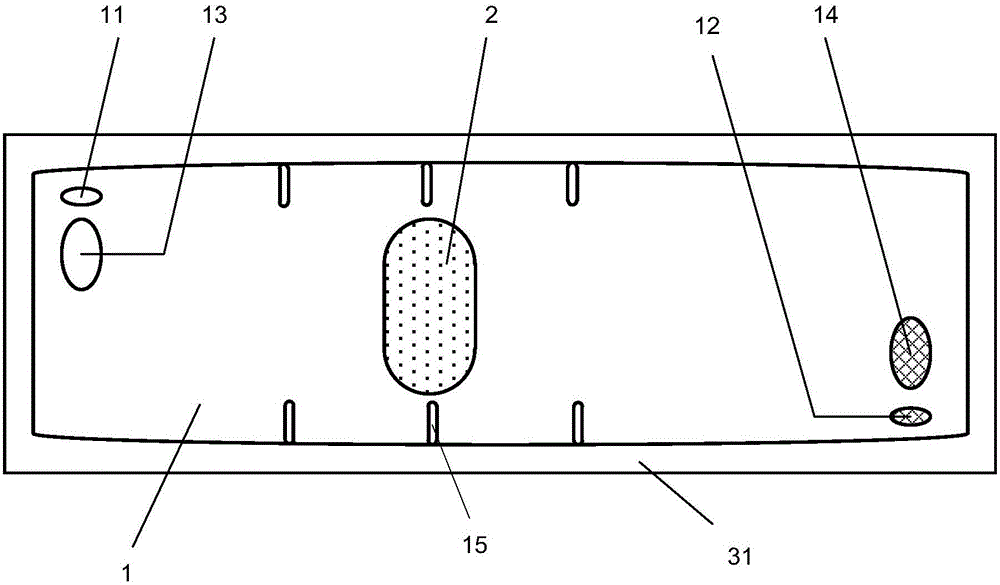
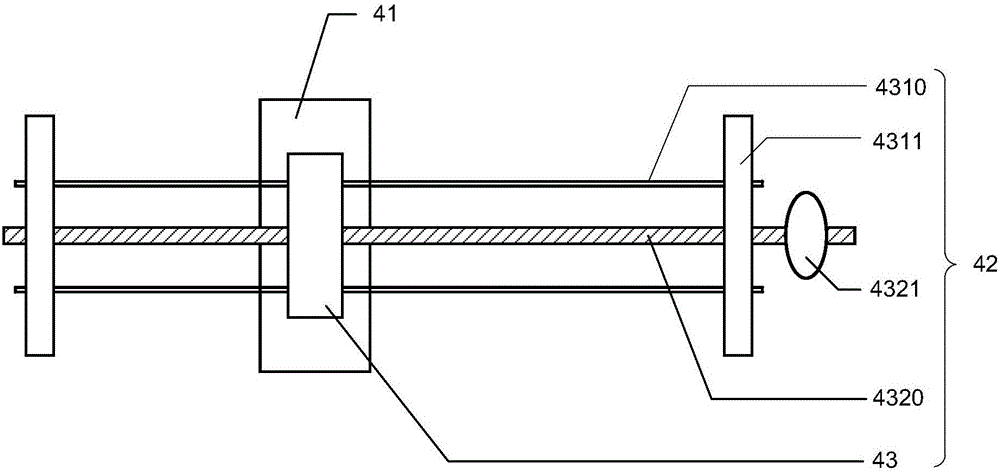
NK cell in-vitro amplification culture medium combination and culture method
An in vitro expansion and NK cell technology, which is applied in the direction of cell culture active agents, tissue cell/virus culture devices, biochemical equipment and methods, etc., can solve unsafe and ethical, cytokine expression changes, and cell culture Safety and other issues, to achieve effective amplification and activation, high activity, and increased safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Prepare basal medium, induction medium, proliferation medium and activation adaptation medium according to the medium composition given in Table 1.
[0091] Healthy volunteers collected 25mL of peripheral blood from the cubital vein on an empty stomach in the morning, added heparin for anticoagulation; used lymphocyte separation medium to perform density gradient centrifugation, collected mononuclear cell layers, and counted A after washing with PBS centrifugation. Mononuclear cells were resuspended, and the cell density was adjusted to 5×10 5 cells / mL, cell culture was carried out in the immune cell in vitro expansion device described in the present disclosure, firstly in 5% CO 2 , the basal culture was carried out for 1.5 days under the environment of 37° C. to obtain the basal culture cells. Collect the cells by centrifugation and adjust the cell density to 5×10 5 cells / mL, in 5% CO 2 , the induction culture was carried out for 3 days under the environment of 37° ...
Embodiment 2
[0098] The method of Example 1 was used to culture NK cells in vitro, the only difference being that the components of each culture medium used are shown in Table 2. The amplification factor and purity results of the detected cells are shown in Table 5 and Figure 4 , 5 As shown, the killing rate results of NK cells are shown in Table 6 and Image 6 shown.
[0099] Table 2
[0100]
Embodiment 3
[0102] The method of Example 1 was used to culture NK cells in vitro, the only difference being that the components of each culture medium used are shown in Table 3. The amplification factor and purity results of the detected cells are shown in Table 5 and Figure 4 , 5 As shown, the killing rate results of NK cells are shown in Table 6 and Image 6 shown.
[0103] table 3
[0104]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com