Method for detecting related substances in irbesartan hydrochlorothiazide tablets by adopting high performance liquid chromatography
A technology of high performance liquid chromatography and chlorothiazide tablets, which is applied in the field of high performance liquid chromatography to detect related substances in irbesartan and hydrochlorothiazide tablets, can solve solvent peak interference, hydrochlorothiazide impurity chlorothiazide cannot be completely separated, and peak shape is poor And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0037] According to the determination method of the irbesartan hydrochlorothiazide tablet of the present invention, the accelerated sample sample of the self-made irbesartan hydrochlorothiazide tablet whose batch number is 20131104 is measured, and the content of each impurity in the related substance item is checked.
[0038] Take 10 samples of the batch number and grind them finely, weigh 150.23mg, put them in a 100ml measuring bottle, add 30ml of solvent, ultrasonicate for 10 minutes, let cool, dilute to the mark with solvent, shake well, filter through; accurately measure 7.5ml of subsequent filtrate , put in a 50ml measuring bottle, dilute to the mark with a solvent, and shake well.
[0039] Reference substance: reference substance solution (a): Accurately weigh 15.14 mg of irbesartan reference substance, accurately weigh it, put it in a 25ml measuring bottle, add 5ml of solvent and ultrasonically dissolve it for 2 minutes, dilute to the mark with solvent, shake well, ins...
experiment example 2
[0054] 1.1 Selection of detection wavelength
[0055]Precisely weigh an appropriate amount of irbesartan and irbesartan A reference substance, dissolve it with the above-mentioned solvent and constant volume, and prepare a mixed reference solution of about 20 μg / ml; accurately weigh an appropriate amount of hydrochlorothiazide reference substance, dissolve it with a solvent and constant volume, Prepare the mixed reference substance solution as a solution of 20 μg / ml, use the solvent as a blank, scan in the wavelength range of 200-400 nm according to the ultraviolet spectrophotometry, and record the ultraviolet absorption spectrum.
[0056] attached by figure 1 Irbesartan and figure 2 Hydrochlorothiazide shows that in the 200-300nm wavelength range, hydrochlorothiazide is at 220nm (see figure 2 There is maximum absorption at 1), 270nm and 312nm in reference sign 1); Irbesartan and its impurity irbesartan A are at 206nm (see figure 1 Reference numeral 1) has the maximum a...
Embodiment 2
[0072] Establishment of methodology
[0073] 2.1 Specificity
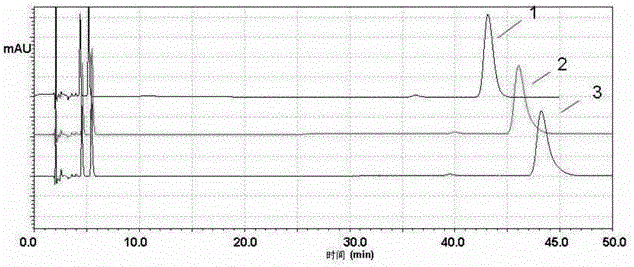
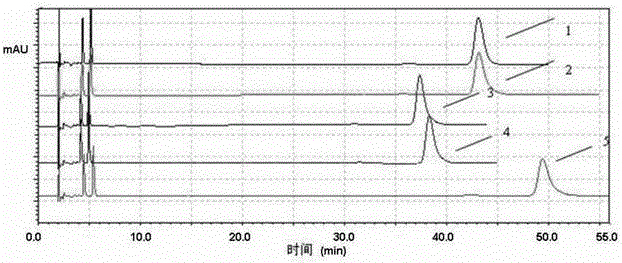
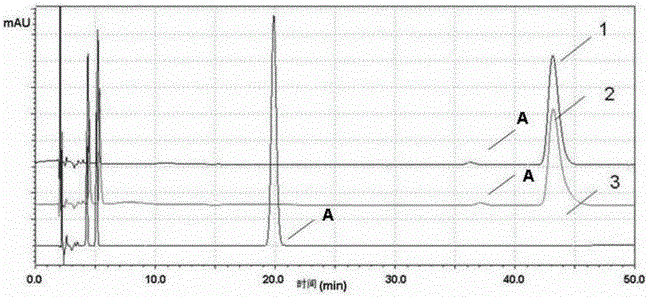
[0074] The preparation of the specific solution is prepared according to the preparation method of the reference substance (2) in this patent, and each 20 μl of the above-mentioned linear solution is accurately measured, injected into the liquid chromatograph, and the chromatogram is recorded, see image 3 . attached image 3 The one marked as 2 is irbesartan, with image 3 The one marked 1 in the figure is irbesartan A, the one marked 3 in the drawings is arginine sulfonamide, and the one marked 4 in the drawings is hydrochlorothiazide.
[0075] Table 3 specific chromatographic separation table
[0076]
[0077] The test results show that the solvent peaks early and does not interfere with the detection of related substances; the impurities mixed in the control solution and the system suitability test solution are shown in the attached image 3 Available, fine sulfonamide (see image 3 Reference numeral 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com