Photo-responsive controlled-release nano-pesticide preparation and preparation method and application thereof
A light-responsive, nano-pesticide technology, applied in the direction of botanical equipment and methods, applications, herbicides and algicides, etc., can solve the problem that the quantity of drugs does not meet the requirements, the time for drug efficacy is not enough, and the adverse effects on humans and the environment To achieve good anti-dilution stability, improve the efficiency of drug action, and reduce the number of application times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Preparation of o-nitrobenzyl succinic acid monoester:
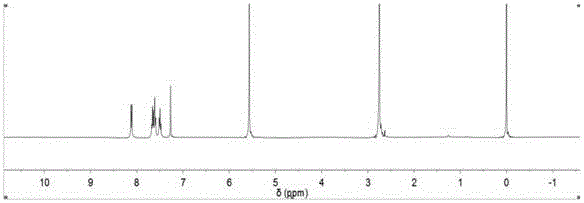
[0044] Weighing a molar ratio of 1:2 o-nitrobenzyl alcohol and succinic anhydride dissolved in chloroform, adding catalyst 4-dimethylaminopyridine (DMAP) in an amount 0.2 times the amount of raw material, under nitrogen protection environment, reflux After reacting for 24 hours, after stopping the reaction, part of the chloroform was distilled off under reduced pressure, and the remaining reaction solution was washed three times with a 10% (v / v) HCl solution by volume percentage, then extracted and washed with a saturated sodium bicarbonate solution, and washed with a volume percentage concentration of The pH of the aqueous phase was adjusted to 5 for a 10% (v / v) HCl solution, and a large amount of white precipitate formed. Collect the white precipitate and dry it under vacuum at 40°C to obtain o-nitrobenzyl succinic acid monoester (NBS).
[0045] The vacuum drying process parameters are: vacuum degree<133Pa, ...
Embodiment 2
[0054] (1) Preparation of o-nitrobenzyl succinic acid monoester:
[0055] With embodiment 1.
[0056] (2) Preparation of o-nitrobenzyl succinic acid monoester DMF solution activated by NHS and EDC HCl:
[0057] Dissolve the o-nitrobenzyl succinic acid monoester in DMF, and stir evenly at room temperature to prepare a 20% by weight o-nitrobenzyl succinic acid monoester DMF solution. The solution was cooled to 0-5°C, according to o-nitrobenzyl succinic acid monoester: N-hydroxysuccinimide (NHS): 1-(3-dimethylaminopropyl)-3-ethylcarbodiethylene Amine hydrochloride (EDC·HCl) is 1:1.1:1.1 in molar ratio, add NHS and EDC·HCl in turn, react at room temperature for 24 hours, and filter the reaction solution to obtain o-nitrobenzyl succinic acid activated by NHS and EDC·HCl Monoester DMF solution.
[0058] (3) Preparation of photoresponsive carboxymethyl chitosan
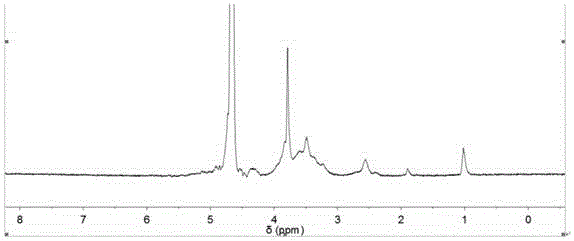
[0059] Take a carboxymethyl chitosan aqueous solution with a carboxymethyl substitution degree of 1.2 and a weight per...
Embodiment 3
[0062] (1) Preparation of o-nitrobenzyl succinic acid monoester:
[0063] With embodiment 1.
[0064] (2) Preparation of o-nitrobenzyl succinic acid monoester DMF solution activated by NHS and EDC HCl:
[0065] Dissolve the o-nitrobenzyl succinic acid monoester in DMF, and stir evenly at room temperature to prepare a 15% o-nitrobenzyl succinic acid monoester DMF solution. The solution was cooled to 0-5°C, according to o-nitrobenzyl succinic acid monoester: N-hydroxysuccinimide (NHS): 1-(3-dimethylaminopropyl)-3-ethylcarbodiethylene Amine hydrochloride (EDC·HCl) is 1:1.1:1.1 in molar ratio, add NHS and EDC·HCl in turn, react at room temperature for 24 hours, and filter the reaction solution to obtain o-nitrobenzyl succinic acid activated by NHS and EDC·HCl Monoester DMF solution.
[0066] (3) Preparation of photoresponsive carboxymethyl chitosan
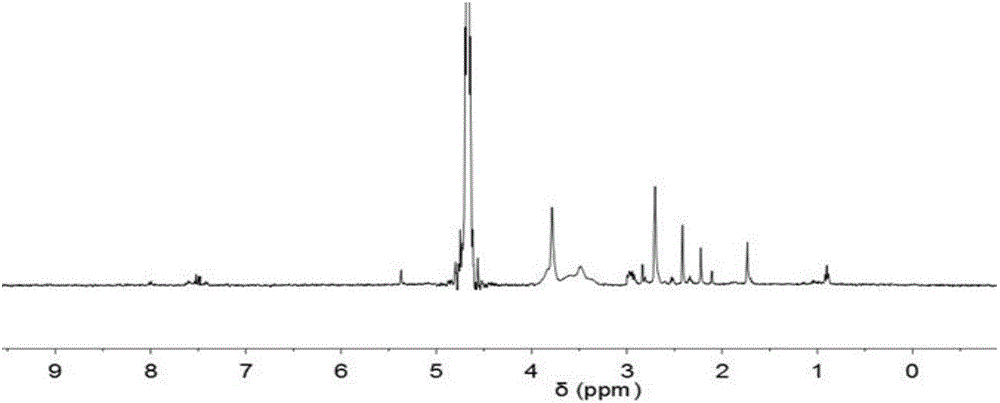
[0067] Take a carboxymethyl chitosan aqueous solution with a carboxymethyl substitution degree of 1.6 and a concentration of 5.0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical micelle concentration (mass) | aaaaa | aaaaa |
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com