Preparation and characterization method of photosensitizer/antitumor drug self-assembled nano drug delivery system based on hyaluronic acid
A nano drug delivery system and anti-tumor drug technology, which is applied in the direction of anti-tumor drugs, drug combinations, medical preparations of non-active ingredients, etc., and can solve problems such as limited application, short half-life, and easy aggregation and photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
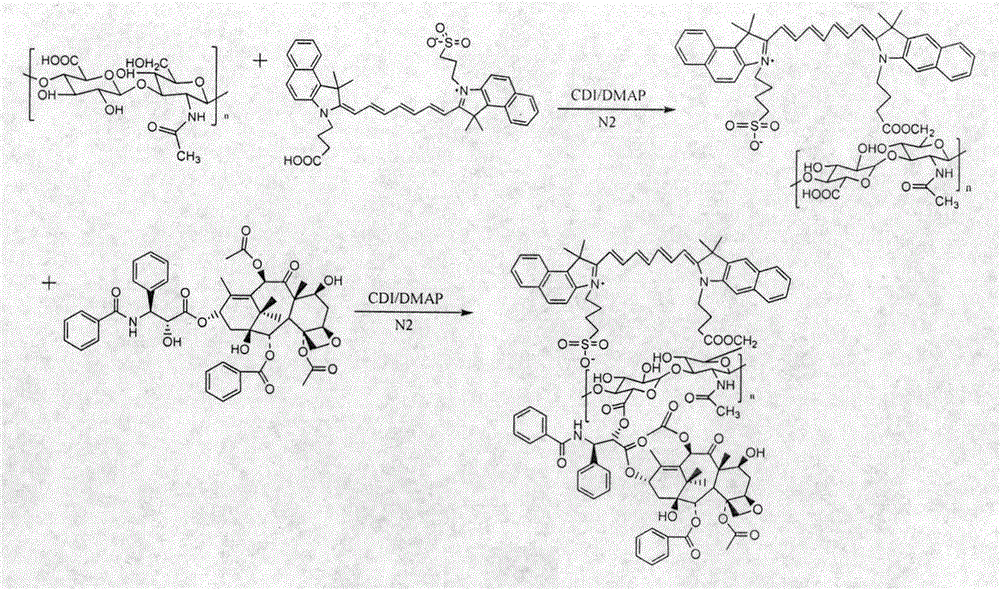
[0026] Embodiment 1: the synthesis of hyaluronic acid-carboxylated indocyanine green (HA-ICG)
[0027] Weigh 46.4mg of carboxylated indocyanine green (ICG-COOH) and 126.4mg of N,N'-dihydroxyimidazole (CDI) and dissolve in 20ml of DMF solution, and stir at room temperature for 0.5h.
[0028] Weigh 100mg of sodium hyaluronate (HA-COONa) and 95.2mg of 4-dimethylaminopyridine (DMAP) and dissolve in 10ml of formamide solution, slowly add the activated carboxylated indocyanine green solution to the HA-COONa solution, N 2 Stir at room temperature under protection for 14h. The reactant was put into a dialysis bag (MWCO: 3500), dialyzed with 50% DMF solution for 24 hours, and then dialyzed with deionized water for 48 hours. Freeze-dry to obtain hyaluronic acid-carboxylated indocyanine green (HA-ICG) (see attached figure 1 ).
Embodiment 2
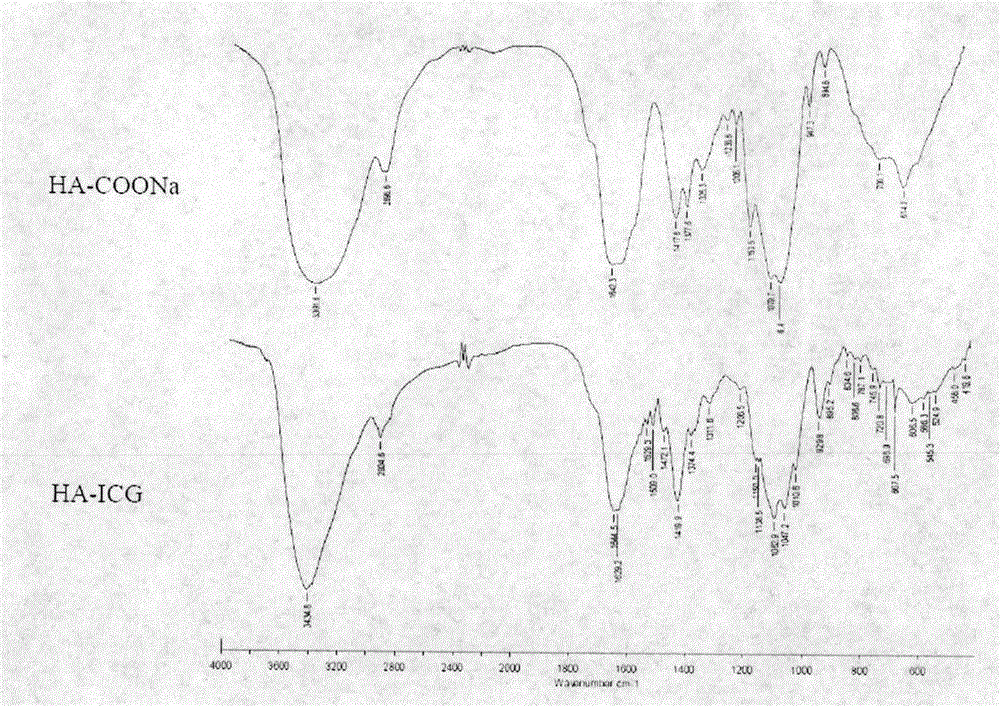
[0029] Example 2: Structural characterization of hyaluronic acid-carboxylated indocyanine green (HA-ICG)
[0030] (1) Infrared spectroscopy (FT-IR) characterization: Take appropriate amount of HA-COONa and HA-ICG, mix with KBr, grind into powder, and press into thin slices. Structural analysis was performed with Fourier transform infrared spectroscopy. FT-IR spectrum (attached figure 2 ) analysis results are as follows: The characteristic absorption peak of HA-COONa is 3391.6cm -1 (v NH ), 1642.3cm -1 (v C=O , amide bond), 2898.6cm -1 (v S CH3 ); and in the HA-ICG spectrum, a new characteristic absorption peak appeared at 1629.2cm -1 (v C=O , ester bond), 1529.3cm -1 、1509cm -1 (conjugated benzene ring), 929.8cm -1 (trans double bond), these are the characteristic peaks of ICG, indicating that ICG has been covalently linked to HA through ester bonds to form HA-ICG conjugates.
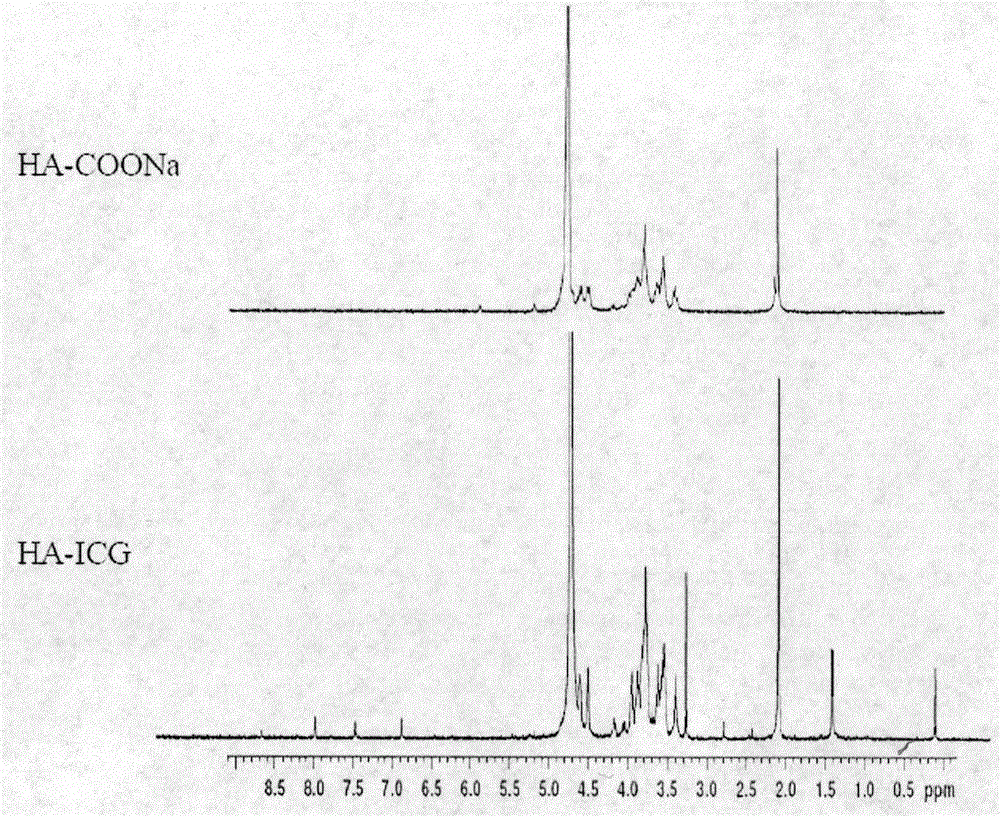
[0031] (2) NMR spectrum ( 1 (H-NMR) characterization: take appropriate amount of HA-C...
Embodiment 3
[0032] Embodiment 3: Synthesis of carboxylated indocyanine green-hyaluronic acid-paclitaxel (ICG-HA-PTX)
[0033] Weigh 75mg of HA-ICG and 49.95mg of N,N'-dihydroxyimidazole (CDI) and dissolve in 20ml of DMF solution, and stir at room temperature for 1h.
[0034] Another 14.06 mg of PTX and 37.5 mg of 4-dimethylaminopyridine (DMAP) were dissolved in 4 ml of DMF solution, and the above-mentioned activated HA-ICG solution was slowly added to the PTX solution, N 2 Stir at room temperature under protection for 24h. The reactant was put into a dialysis bag (MWCO: 3500), dialyzed with DMF solution for 24 hours, and then dialyzed with deionized water for 48 hours. Freeze-drying to obtain carboxylated indocyanine green-hyaluronic acid-paclitaxel (ICG-HA-PTX) (see attached figure 1 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com