Method for preparing nickel chloride through sulfuric acid leaching of crude nickel hydroxide
A technology of nickel hydroxide and nickel chloride, which is applied in the field of hydrometallurgy, can solve problems such as high impurity content of nickel chloride, surrounding environmental pollution, and easy leakage of chlorine gas, and achieve high product quality, strong extraction ability, and chlorine gas reduction. The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
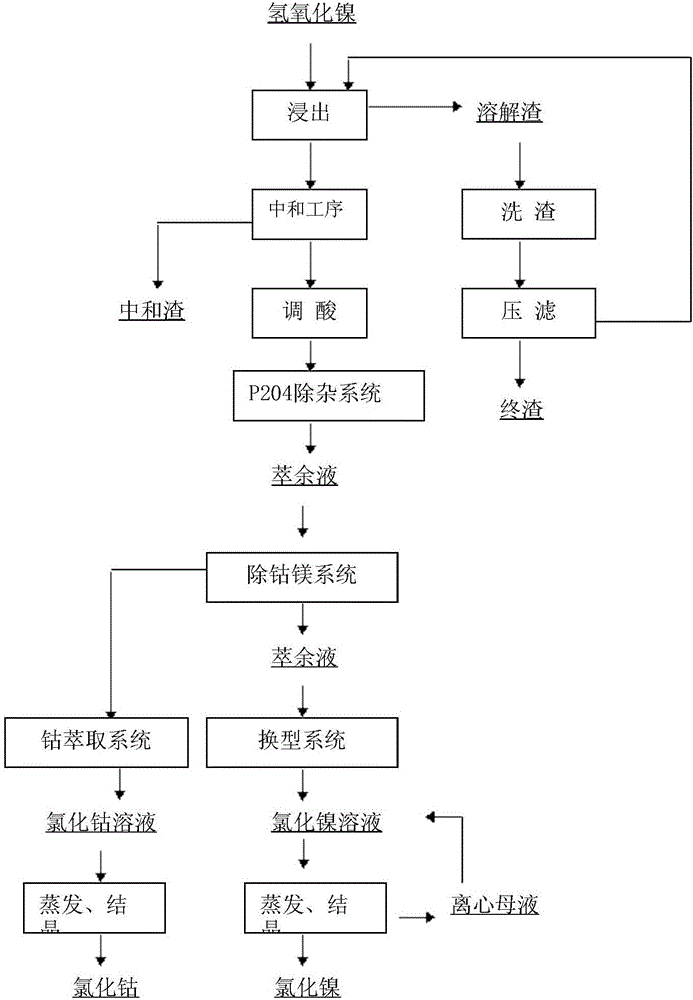
[0024] Such as figure 1 As shown, the present invention proposes a kind of preparation method that takes crude nickel hydroxide as raw material sulfuric acid leaching to produce nickel chloride, it is characterized in that, the method comprises the steps:
[0025] Step 1, the sulfuric acid leaching process of nickel hydroxide comprises the following sub-steps:
[0026] Put the crude nickel hydroxide into the leaching tank, slowly add 93% concentrated sulfuric acid, control the speed of adding concentrated sulfuric acid to add 1.4L-1.5L concentrated sulfuric acid every 4 hours, control the temperature at 60°C-80°C, adjust the pH value to 1.0 to 2.0, to obtain ore pulp, separate the leached ore pulp from liquid to solid to obtain leaching slag and leachate; the leaching slag enters the slag washing tank and is washed with water to reduce the final slag nickel;
[0027] The leachate is transported to the neutralization tank, the temperature is raised to 60°C-80°C, sodium carbona...
Embodiment 1
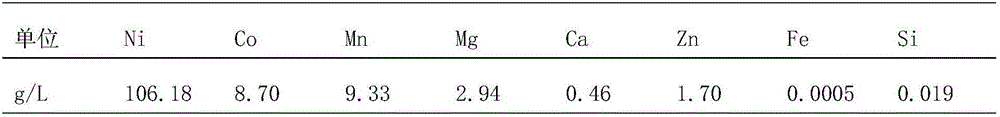
[0040] Put 10kg of crude nickel hydroxide into the experimental leaching tank, control the speed of adding 93% concentrated sulfuric acid, adjust the pH value of the pulp to 1.3, and pump the filtrate into the neutralization tank after the pulp is press-filtered, raise the temperature to 80°C, and slowly add sodium carbonate Solid to pH 5.2, add 93% concentrated sulfuric acid, and the index of the liquid after neutralization is shown in the table below:
[0041]
[0042] Adjust the pH value of the neutralized liquid to 3.58 and send it to the extraction experiment system.
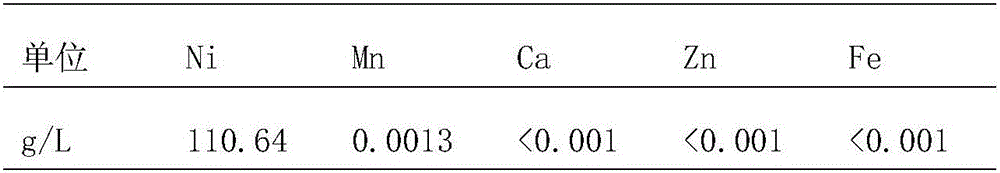
[0043] The flow rate of P204 extraction organic phase is 2.5L / h, the flow rate of lye is 0.2L / h, the flow rate of stock solution is 1.6L / h, the flow rate of washing acid is 0.15L / h, and the flow rate of stripping acid is 0.15L / h. Raffinate indicators are shown in the table below:
[0044]
[0045]The flow rate of P507 extraction agent is 2.6L / h for organic phase, 0.25L / h for lye, 1.6L / h for stock sol...
Embodiment 2
[0052] Put 10kg of crude nickel hydroxide into the experimental leaching tank, control the speed of adding 93% concentrated sulfuric acid, the pH value of the pulp reaches 1.27, after the pulp is filtered by pressure, the filtrate is pumped into the neutralization tank, the temperature is raised to 80 °C, and sodium carbonate solid is slowly added The pH value is 5.36, add 93% concentrated sulfuric acid, and the index of the liquid after neutralization is shown in the table below:
[0053]
[0054] Adjust the pH value of the neutralized solution to 3.67 and send it to the extraction experiment system.
[0055] The flow rate of P204 extraction organic phase is 2.5L / h, the flow rate of lye is 0.19L / h, the flow rate of stock solution is 1.5L / h, the flow rate of washing acid is 0.15L / h, and the flow rate of stripping acid is 0.15L / h. Raffinate indicators are shown in the table below:
[0056]
[0057] The flow rate of P507 extraction agent is 2.6L / h for organic phase, 0.26L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com