Method for synthesizing TTZ (thiotriazinone) by using self-made organic base catalyst taking graphene as carrier
A base catalyst and graphene technology, applied in organic compound/hydride/coordination complex catalysts, organic chemistry, chemical instruments and methods, etc., can solve the problem of high production cost of triazine rings, refractory or processing costs, partial Concentration unevenness and other problems, to achieve the effect of reducing environmental protection investment, reducing the amount of waste, and easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
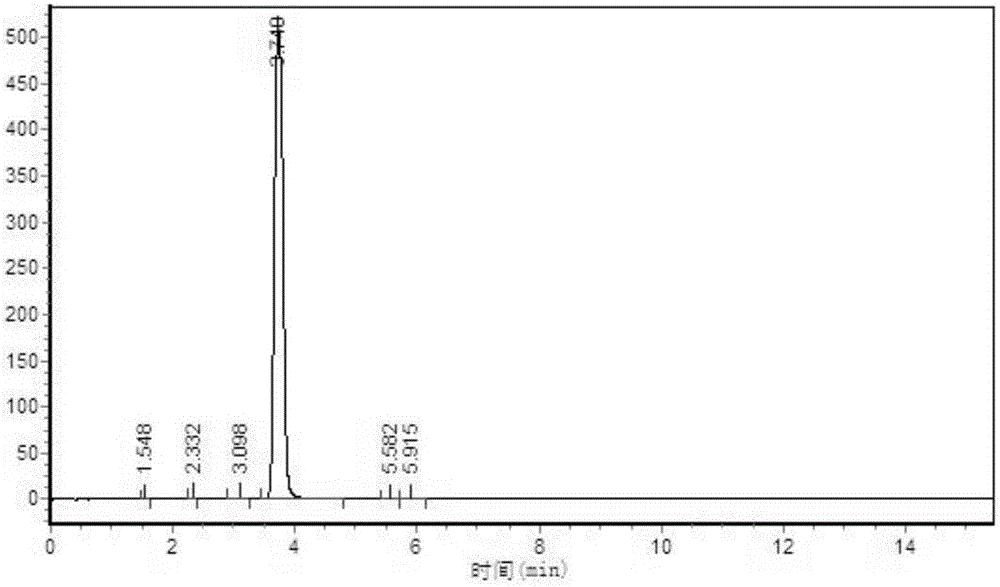
[0022] Add 180kg of 2-methylthiosemicarbazide, 315kg of diethyl oxalate, 1260kg of methanol solvent, 228kg of self-made alkali catalyst with graphene as the carrier, and carry out ring closure reaction at 0-60°C. After the reaction is completed, decolorize , acidified with hydrochloric acid, filtered and dried to obtain a triazine ring with a yield of 85.8% and a by-product of 85 ppm. The HPLC spectrum of the triazine ring is as follows figure 1 As shown, the small peak 3 minutes before the main peak is the peak of the by-product 1-methyl-5-mercapto-1,2,4-triazole-3-carboxylic acid methyl ester.
Embodiment 2
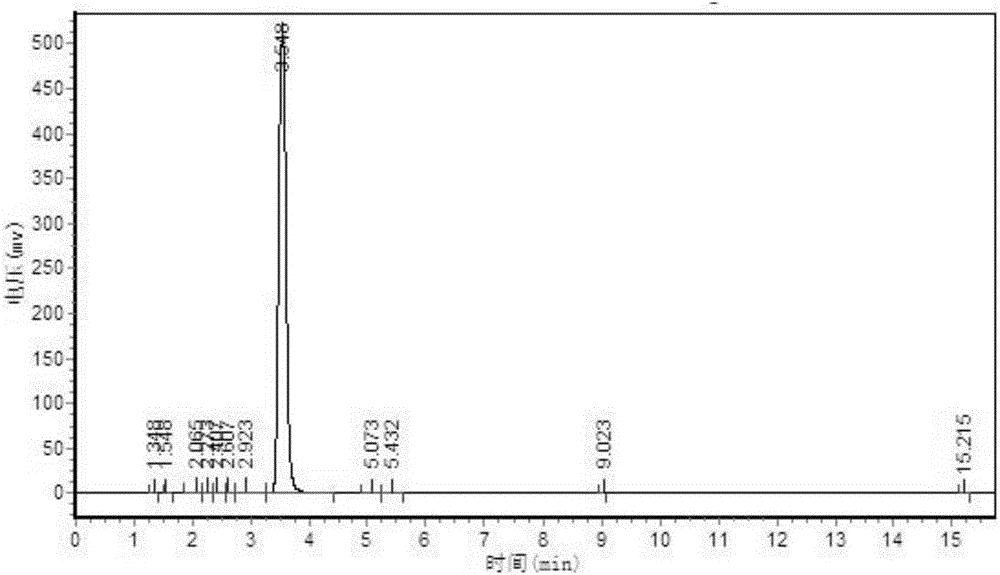
[0024] Add 180kg of 2-methylthiosemicarbazide, 270kg of diethyl oxalate, 1080kg of methanol solvent, 230kg of self-made alkali catalyst with graphene as the carrier, and carry out ring closure reaction at 0-60°C. After the reaction is completed, decolorize 1. After acidifying with hydrochloric acid, filter and dry to obtain a triazine ring with a yield of 85.2% and a by-product of 90 ppm. The HPLC spectrum of the triazine ring is as follows figure 2 As shown, the small peak 3 minutes before the main peak is the peak of the by-product 1-methyl-5-mercapto-1,2,4-triazole-3-carboxylic acid methyl ester.
Embodiment 3
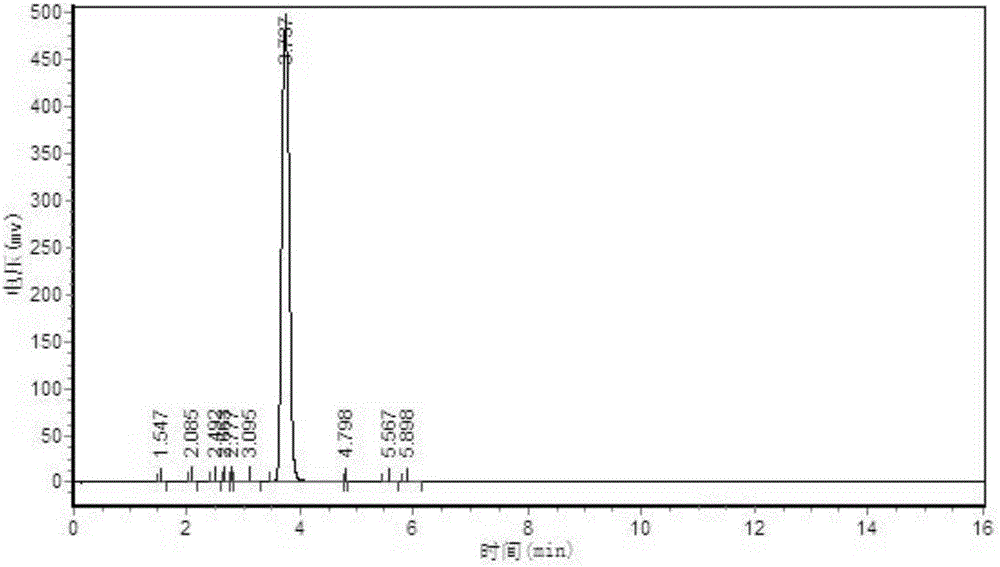
[0026] Add 180kg of 2-methylthiosemicarbazide, 360kg of diethyl oxalate, 2160kg of methanol solvent, 270kg of self-made alkali catalyst with graphene as the carrier, and carry out ring closure reaction at 0-60°C. After the reaction is completed, decolorize 1. After acidifying with hydrochloric acid, filter and dry to obtain a triazine ring with a yield of 85.6% and a by-product of 95 ppm. The HPLC spectrum of the triazine ring is as follows image 3 As shown, the small peak 3 minutes before the main peak is the peak of the by-product 1-methyl-5-mercapto-1,2,4-triazole-3-carboxylic acid methyl ester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com