Zone structured terpolymer based on monofluoro-substituted benzoheterocycle and application thereof
A terpolymer, heterocycle technology, applied in electrical components, photovoltaic power generation, circuits, etc., to achieve good planarity and rigidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
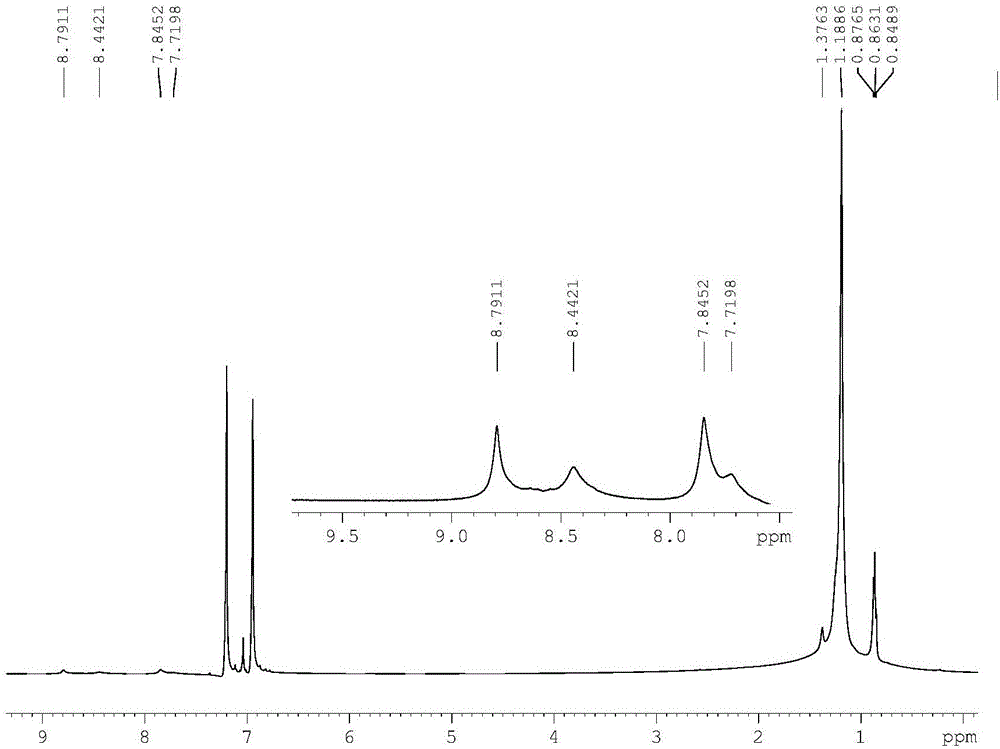
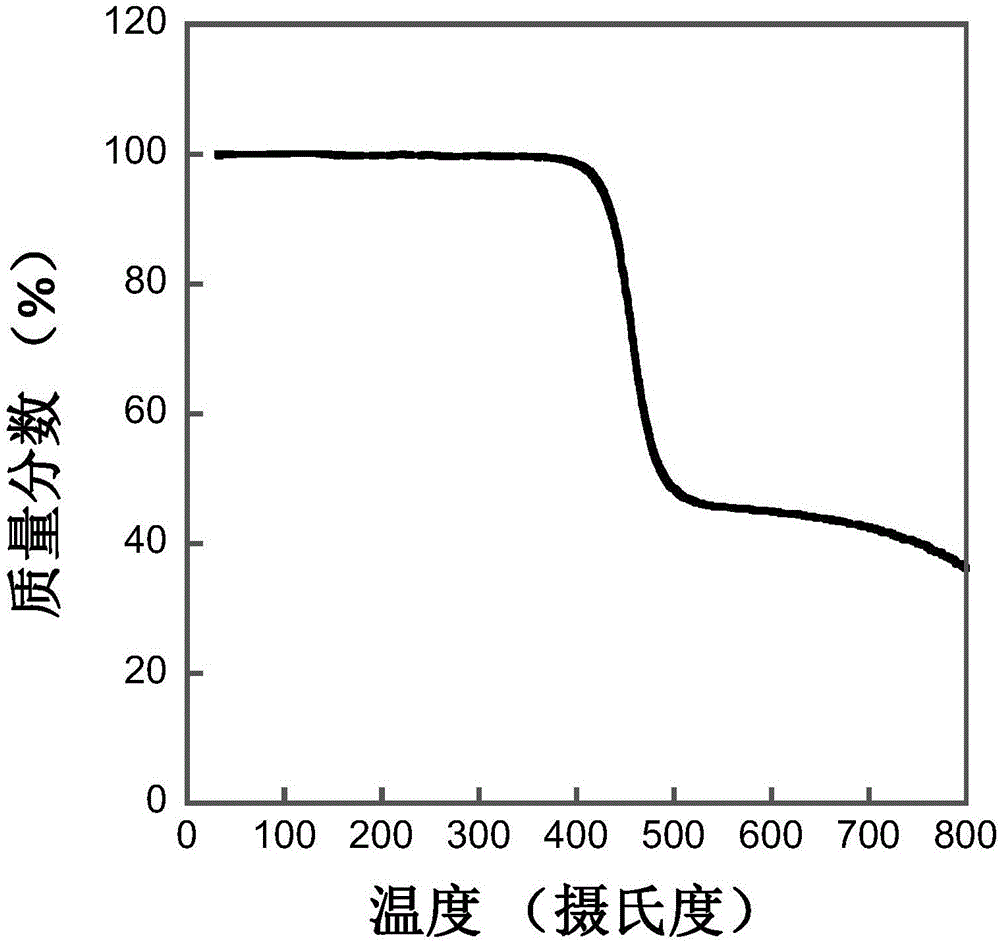
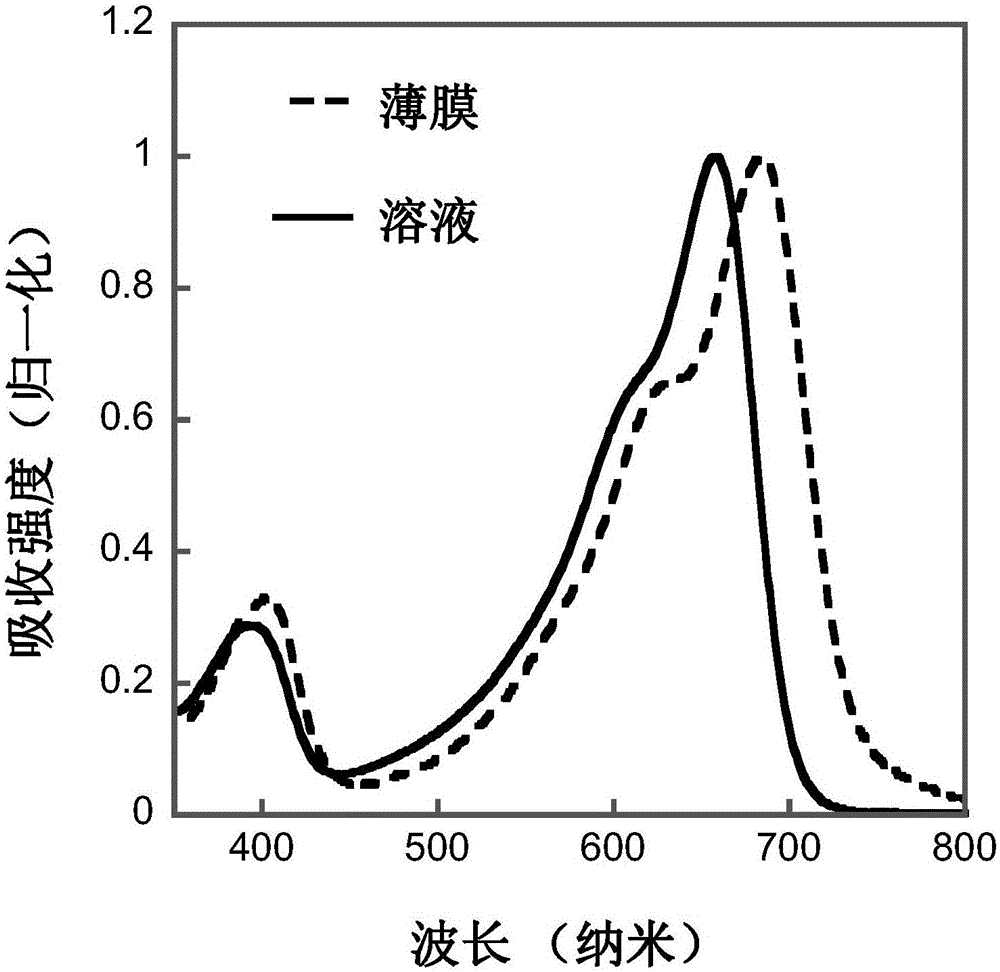
[0040] Based on the regioregular terpolymer of indaprodithiophene, dithiophene and 5-fluorobenzo[d][1,2,5]thiadiazole (abbreviated as IFBT-TT)
[0041] The synthetic route is as follows:
[0042]
[0043] (1) 2,7-bis(trimethyltin)-4,9-dihydro-4,4,9,9-hexadecyl-s-indacene[1,2-b:5, Preparation of 6-b']dithiophene (Compound 1)
[0044]Under argon atmosphere, in a 100mL two-necked round bottom flask, 4,9-dihydro-4,4,9,9-hexadecyl-s-indacene[1,2-b:5, 6-b'] Dithiophene (0.5 g, 0.43 mmol) was dissolved in anhydrous tetrahydrofuran (50 mL). Place the two-necked bottles in a cold well, and after the temperature drops to -78°C, slowly drop n-butyllithium (0.9mL, 1.6mol L -1 , hexane solution), the reaction was kept at -78°C and stirred for 15 minutes; the cold well was removed, the temperature of the reaction solution rose to room temperature, the reaction bottle was placed in an oil bath, and after the temperature was heated to 40°C, stirred for 1 hour; subsequently, Add trimeth...
Embodiment 2
[0054] Based on the regioregular terpolymer of indaprodithiophene, bithiophene and 5-fluorobenzo[d][1,2,5]thiadiazole (abbreviated as IFBT-T-T)
[0055] The synthetic route is as follows: (2,7-bis(4-bromo-5-fluoro-7-benzo[c][1,2,5]thiadiazole)-4,9-dihydro-4,4,9 , the preparation of 9-hexadecyl-s-indapro[1,2-b:5,6-b']dithiophene is the same as in Example 1)
[0056]
[0057] In a water-oxygen automatic control glove box, 5,5'-bis(trimethyltin)-2,2'-bithiophene (36mg, 0.074mmol), 2,7-bis(4-bromo-5-fluoro -7-Benzo[c][1,2,5]thiadiazole)-4,9-dihydro-4,4,9,9-hexadecyl-s-indacene[1,2 -b: Preparation of 5,6-b']dithiophene (compound 2, 120mg, 0.074mmol) and tetrakistriphenylphosphine palladium (4.3mg, 0.0037mmol) were added to a 10mL microwave tube, and anhydrous xylene was added (2.5 mL) was dissolved and the silicone cap was sealed. Place the microwave tube in a microwave reactor and set the temperature program: 80°C for 2 minutes, 120°C for 2 minutes, 160°C for 2 minutes, and ...
Embodiment 3
[0060] Based on the regioregular terpolymer of indaprodithiophene, trithiophene and 5-fluorobenzo[d][1,2,5]thiadiazole (abbreviated as IFBT-DTT)
[0061] The synthetic route is as follows: (2,7-bis(4-bromo-5-fluoro-7-benzo[c][1,2,5]thiadiazole)-4,9-dihydro-4,4,9 , the preparation of 9-hexadecyl-s-indapro[1,2-b:5,6-b']dithiophene is the same as in Example 1)
[0062]
[0063] In a water-oxygen self-control glove box, 2,6-bis(trimethyltin)dithieno[3,2-b:2',3'-d]thiophene (39mg, 0.074mmol), 2,7 -Bis(4-bromo-5-fluoro-7-benzo[c][1,2,5]thiadiazole)-4,9-dihydro-4,4,9,9-hexadecyl- s-indapro[1,2-b:5,6-b']dithiophene (compound 2, 120mg, 0.074mmol) and tetrakistriphenylphosphine palladium (4.3mg, 0.0037mmol) were added to 10mL microwave To the tube, anhydrous xylene (2.5 mL) was added to dissolve, and the silica gel cap was sealed. Place the microwave tube in a microwave reactor and set the temperature program: 80°C for 2 minutes, 120°C for 2 minutes, 160°C for 2 minutes, and finally...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com