Glutamate decarboxylase mutant with improved thermal stability and application thereof
A technology of glutamic acid decarboxylase and thermal stability, applied in the field of bioengineering, can solve problems such as unfavorable GAD application, and achieve the effect of improving the stability of enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 Construction of glutamic acid decarboxylase GAD recombinant bacteria
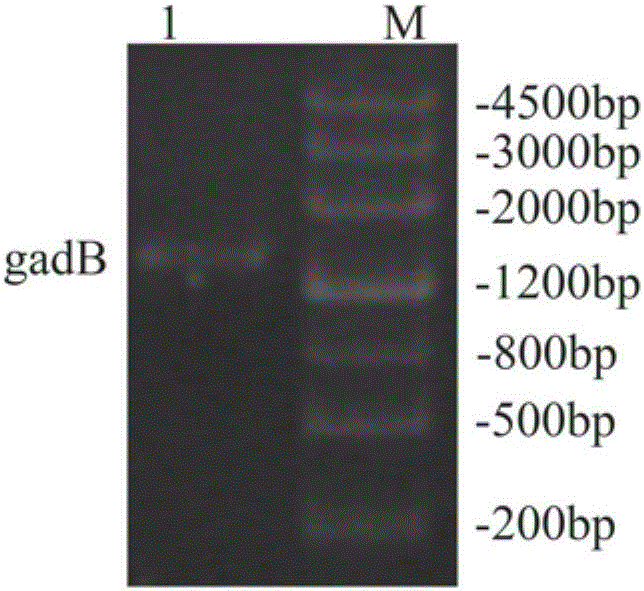
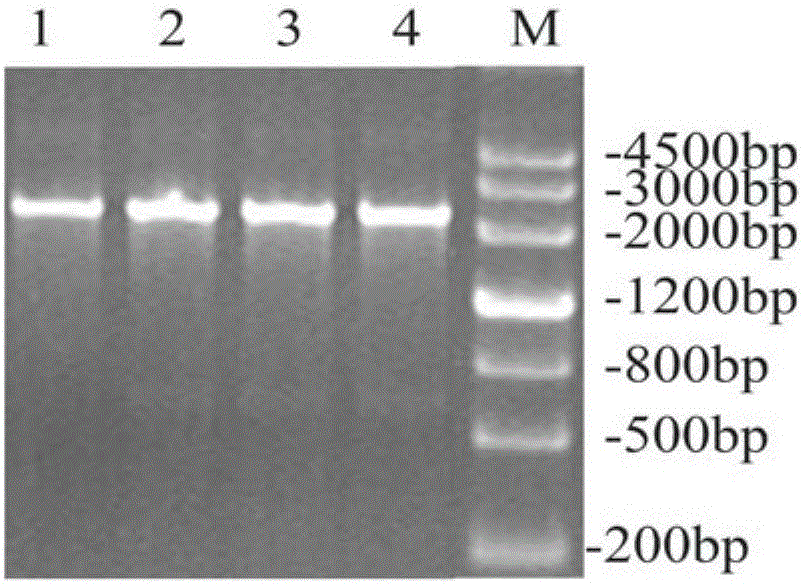
[0040] Using Escherichia coli DH5α genomic DNA as a template, the gadB gene sequence was amplified by PCR using primers 5'-AATTGGATCCATGGATAAGAAGCAAG-3' and 5'-AAGGCTCGAGGGTATGTTTAAAGCTG-3' ( figure 1 ). figure 1 It was shown that the target gene of about 1400bp was successfully obtained. The PCR product and vector pET28a(+) were digested with restriction endonucleases Nco I and Xho I. After the digested product was purified, T4 ligase was ligated overnight at 16°C, transformed into E.coli DH5α competent cells, picked out a single clone and cultured, extracted the plasmid, and identified the presence of gadB with BamH I and Xho I double restriction plasmids ( figure 2 ). figure 2 It showed that the two recombinants picked had a gadB target gene band of about 1400bp and a pET28a(+) carrier band of about 5300bp, both of which were positive clones. The 1# recombinant was selected for ...
Embodiment 2G
[0041] Construction of embodiment 2GAD mutant recombinant bacteria
[0042] Using the plasmid pET28-GADB as a template, the primers 5'-ACCATGGATAAGAAGNNNNNNNNNGATTTAAGGTCGGAAC-3' and 5'-NNNNNNNNNNCTTCTTATCCATGGTATATCTCCTTC-3' were used to amplify the entire plasmid sequence containing the gadB gene by reverse PCR. After digesting with Dpn I, it was transformed into E.coli BL21( DE3) Competent cells, selected monoclonal bacteria, cultured overnight in a 96-well deep-well plate in LB medium containing kanamycin.
Embodiment 3
[0043] The screening of the glutamic acid decarboxylase recombinant bacterium that embodiment 3 thermostability improves
[0044] Transfer the bacterium in Example 2 to a new 96-well deep-well plate containing kanamycin-containing LB medium, culture at 37°C for 2-4h until the OD600 is about 0.6, and add IPTG with a final concentration of 0.2mM , induced overnight at 16°C. Centrifuge to remove the culture medium, add 200ul of 10mM acetic acid-sodium acetate solution containing 20mM bromocresol green, 10mM sodium glutamate, 0.1mMPLP, 10mM pH4.8, incubate at 45°C for 1h, and screen the recombinant strains with dark blue color in the wells. The nucleotide and amino acid sequences of the corresponding plasmid mutation sites were analyzed by sequencing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com