A kind of high temperature resistant α-amylase and its preparation method and application
A technology of high temperature resistance and amylase, applied in the field of genetic engineering, can solve problems such as reducing economic benefits and increasing process complexity, and achieve the effects of reducing energy consumption, obvious economic and social benefits, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Obtaining of wild-type high temperature resistant α-amylase gene
[0030] (1) Extraction of Bacillus licheniformis genomic DNA:
[0031] ①Cultivate the bacteria at 37°C, 200 r / min for 18-24 hours, and collect the bacteria.
[0032] ② Add 500 μL ddH 2 O, resuspend the cells, wash the cells, centrifuge at 5000 r / min for 5 min, and discard the supernatant.
[0033] ③ Add 500 μL ddH 2 O, resuspend the bacteria, add lysozyme (15 μg / mL), and digest in a water bath at 37°C for 1 h.
[0034] ④ Add 50 μL of 10% SDS and 30 μL of proteinase K, and digest in a water bath at 60°C for 2 h.
[0035] ⑤ Add 10% volume of 5 mol / L NaCl, transfer the supernatant tube to add an equal volume of phenol: chloroform: isoamyl alcohol (25:24:1), invert the centrifuge tube several times, and centrifuge at 12000 r / min for 10 min.
[0036] ⑥ Take the supernatant, add an equal volume of phenol: chloroform: isoamyl alcohol (25:24:1), invert the centrifuge tube several times, and centri...
Embodiment 2
[0046] Example 2: Site-directed mutation of the wild-type high temperature resistant α-amylase gene
[0047] (1) The wild-type thermostable α-amylase gene was ligated into the vector pUC19.
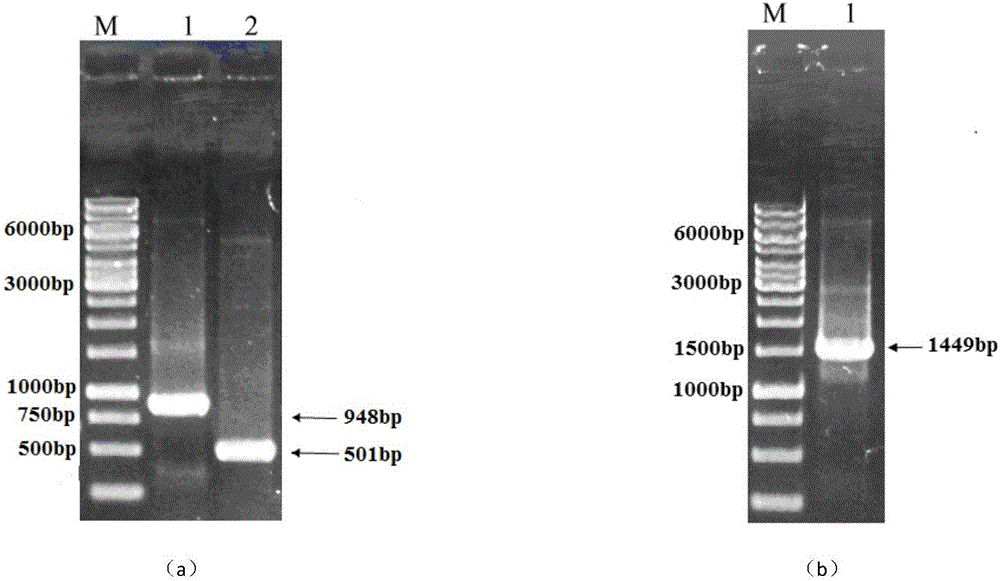
[0048] The target gene obtained by PCR amplification was purified with Bam HI and Hin Digested with dIII, the digested product was purified and detected by agarose gel electrophoresis. At the same time, the plasmid pUC19 was also used Bam HI and Hin dIII was subjected to double digestion, purification, and finally T 4 DNA ligase was ligated at 12°C for 8 hours to construct the recombinant plasmid pUC19- amy . The recombinant plasmid was transformed into Escherichia coli JM109 by electroporation method, and the results of double enzyme digestion and PCR verification showed that amy The gene has been successfully cloned into the vector pUC19.
[0049] Its sequencing shows that it is amplified to the wild-type high temperature resistant α-amylase gene sequence such as SEQ ID N...
Embodiment 3
[0064] Example 3: Construction of a novel high temperature resistant α-amylase expression vector
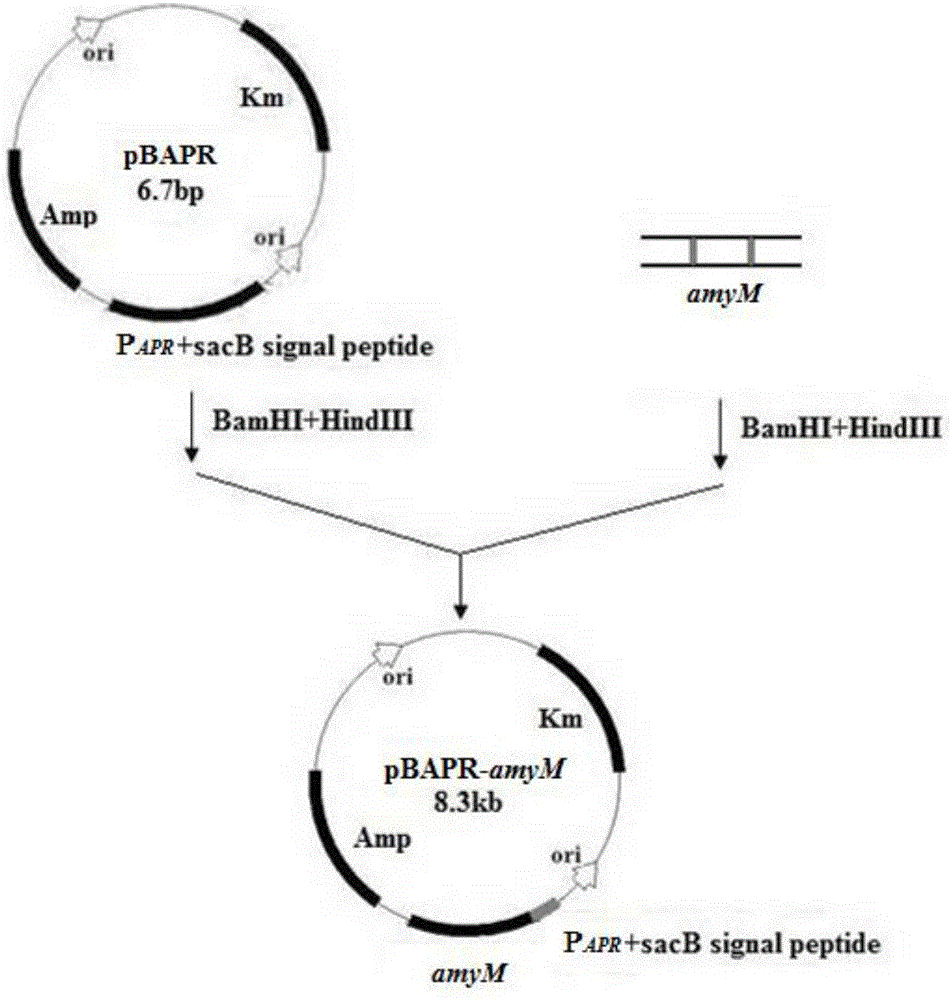
[0065] pBAPR is based on the Escherichia coli-Bacillus subtilis shuttle cloning vector pBE2 as the backbone, cloned into a strong promoter of Bacillus alkalophilus alkaline protease P APR and a fructan sucrase signal sequence that enables direct secretion of recombinant proteins into the culture medium sacB And get. It contains both the replicon of the Bacillus subtilis plasmid pUB110 and the replicon of the Escherichia coli plasmid pGEM3, and can autonomously replicate in Escherichia coli, Bacillus subtilis and Bacillus licheniformis cells. it comes with amp r and Km r Gene, ampicillin resistance can be used as a selection marker in Escherichia coli, and kanamycin resistance can be used as a selection marker in Bacillus subtilis and Bacillus licheniformis.
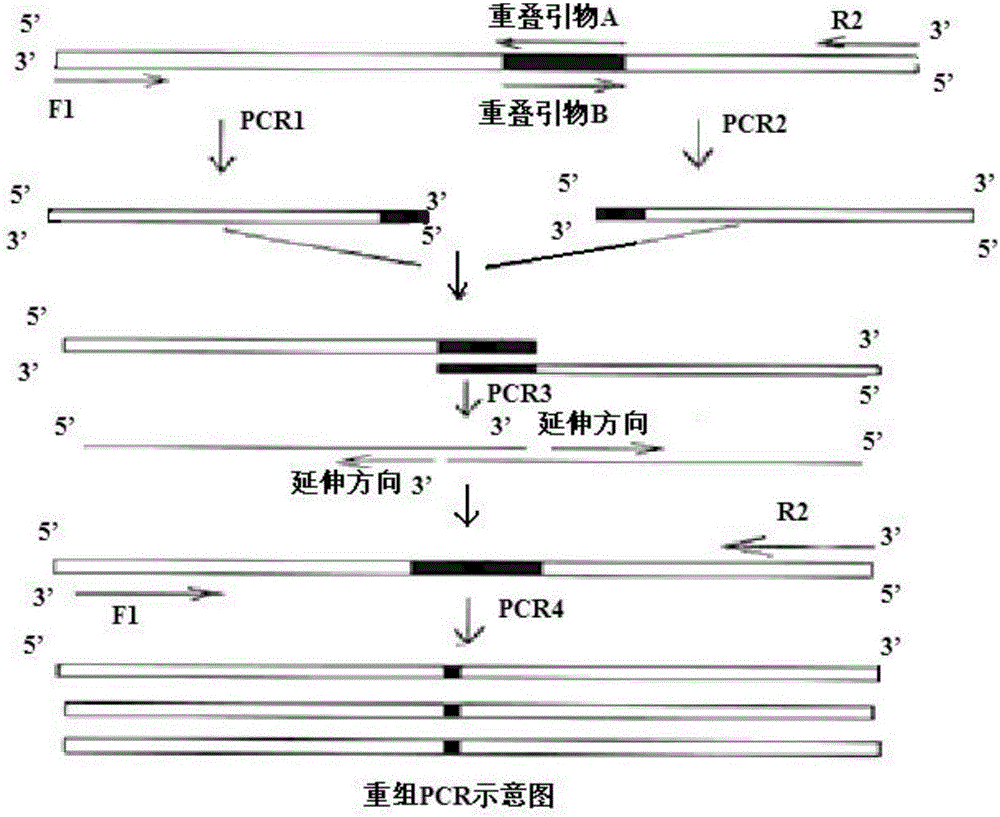
[0066] A new type of high temperature resistant α-amylase gene obtained by overlapping PCR construction amyM with p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com