Optimized glutamine transaminase gene and leader sequence as well as secretory expression thereof
A transaminase gene and glutamine technology, applied in the field of genetic engineering, can solve the problems of low enzyme activity level, expression level, low enzyme activity level, and increase the cost of separation and purification, and achieve the effect of high secretion expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The optimal design and synthesis of embodiment 1 transglutaminase gene and its leader sequence
[0035] 1. Optimal design of transglutaminase gene and its leader sequence
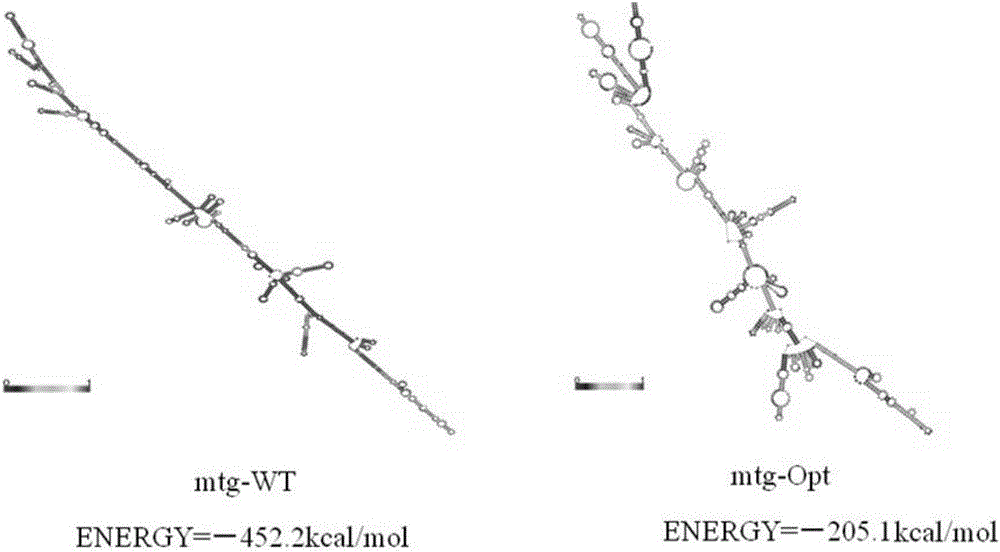
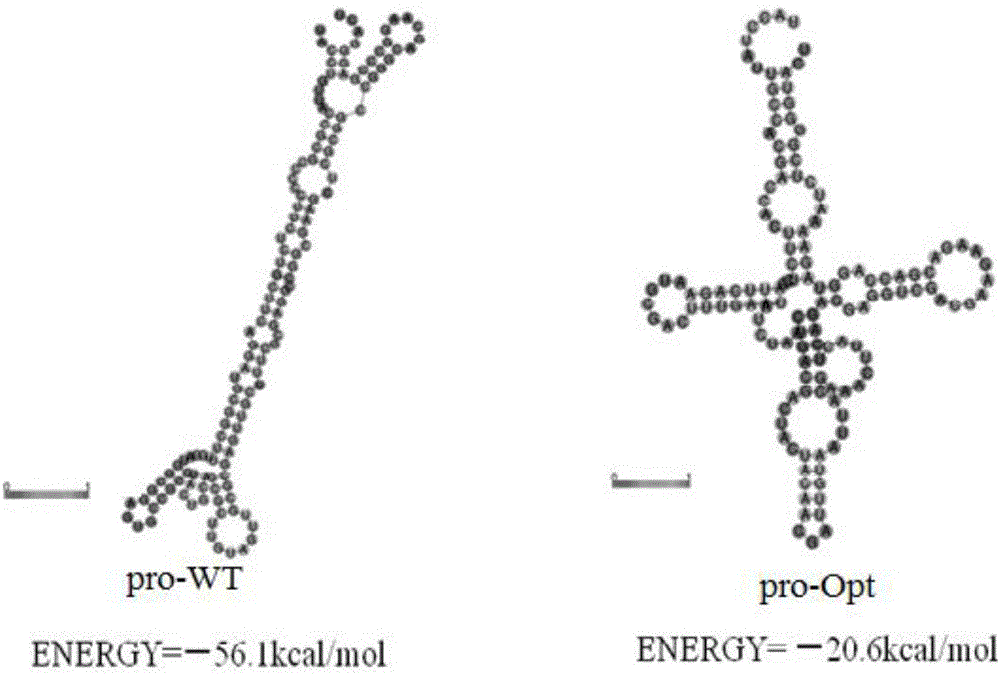
[0036] The present invention first analyzes the sequence of the original gene mtg of transglutaminase mature enzyme cloned from Streptomyces maoyuan and its original leader sequence gene pro, and comprehensively considers the frequency of codon usage without changing the amino acid sequence of the protein , the adjustment of GC content, the deletion of unstable sequences, the stability of mRNA secondary structure and other influencing factors, the transglutaminase gene sequence was modified according to the preferred codons of Pichia pastoris.
[0037] The pro-gene mtg-WT of the mature enzyme of glutamine transaminase derived from Streptomyces Maoyuan, with a full length of 999bp, the sequence is shown in SEQID NO.2, encoding a total of 331 amino acids; the original leader sequence gene pro-WT, with ...
Embodiment 2
[0049] Embodiment 2 Construction of transglutaminase gene and its leader sequence expression vector
[0050] One, the construction of transglutaminase gene (mtg-WT, mtg-Opt) expression vector
[0051] The construction method of the rDNA-mediated multi-copy Pichia pastoris expression vector provided by the present invention first adopts overlapping PCR technology to fuse the AOX1terminator fragment and the rDNA fragment on the carrier pPICZα-B to construct a fusion gene (AOXIterminator-rDNA). Then, the fusion gene fragment was connected with vectors pPICZa-mtg-WT and pPICZa-mtg-Opt (constructed in advance) to obtain expression vectors pPICZα-rDNA-mtg-WT and pPICZα-rDNA-mtg-Opt. Specific steps are as follows:
[0052] (1) Amplification of AOX1 terminator-rDNA fusion gene
[0053] 1. AOX1 terminator amplification: use pPICZα-B as template, Fw-AOX1 and Rv-AOX1 as primers to amplify the end fragment of AOX1 terminator, the size is about 410bp, the 5' end introduces the NotI restr...
Embodiment 3
[0068] Example 3 Expression and purification of glutaminase leader sequence (pro-WT and pro-Opt)
[0069] 1. Construction of recombinant bacteria pro-WT / GS115 and pro-Opt / GS115
[0070] The HIS4 site on the pGAP9-pro-WT and pGAP9-pro-Opt recombinant expression vectors can undergo homologous recombination with the HIS4 site on the yeast GS115 chromosome. SalI is located in the HIS4 region of the pGAP9 plasmid. Recombinant expression vectors pGAP9-pro-WT and pGAP9-pro-Opt were digested with SalI, recovered by cleaning up to obtain linearized plasmids, and electrotransformed into Pichia pastoris GS115 competent cells. Positive clones were screened with MD plates and positively transformed The sons are recombinant bacteria pro-WT / GS115 and pro-Opt / GS115.
[0071] 2. Screening of positive expression clones of recombinant bacteria pro-WT / GS115 and pro-Opt / GS115
[0072] The positive clones pro-WT / GS115 and pro-Opt / GS115 were expanded and cultured using 50mL LA, and the plasmids p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com