One-pot method for preparing hydrophilic phytosterol/stanol derivative
A technology of phytostanols and phytosterols, which is applied in the field of preparation of hydrophilic phytosterols/stanols derivatives, can solve the problems of long reaction time and low product conversion rate, achieve low cost, high product purity, and improve Effects on Industrial Production Levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
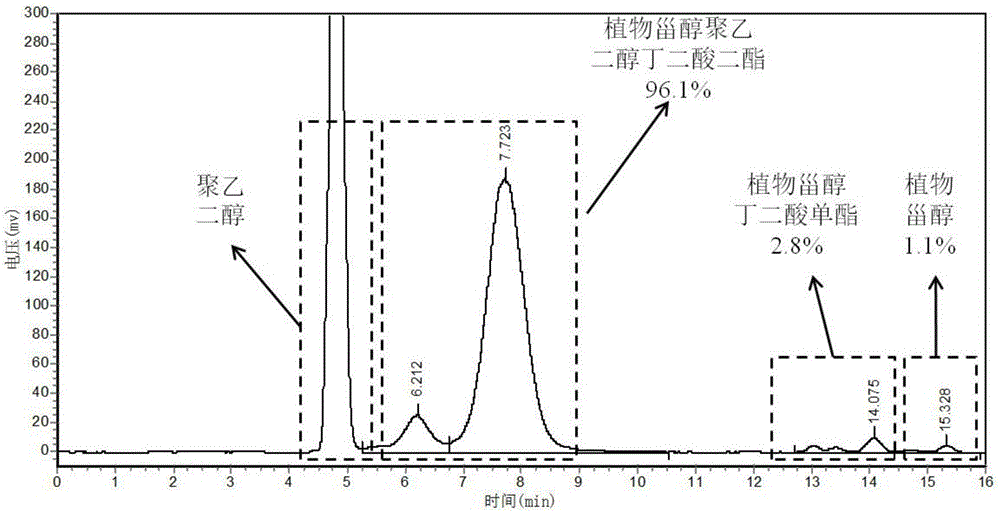
[0038] Embodiment 1 Phytosterol polyethylene glycol succinate diester
[0039] 1. Preparation of phytosterol succinate monoester
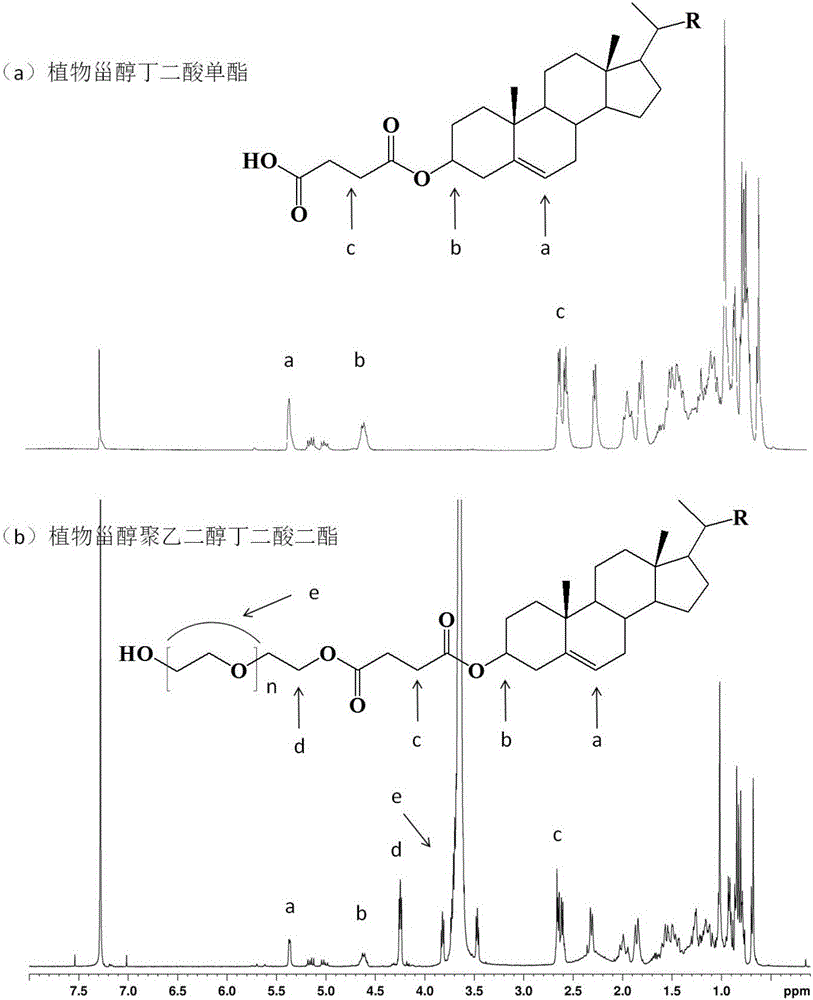
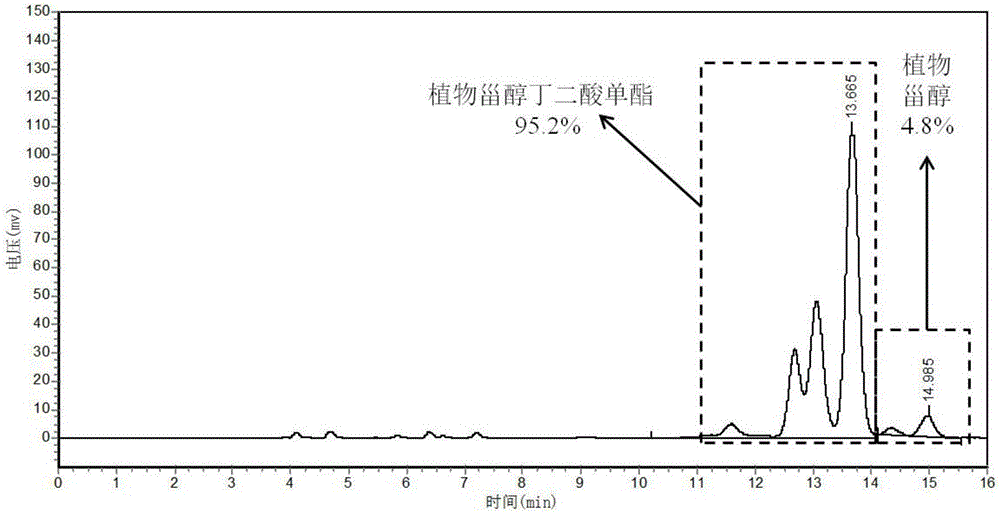
[0040] Preparation method: Add 12.4g phytosterols, 3.0g succinic anhydride, and 0.25g ionic liquid 1-butylsulfonic acid-3-methylimidazolium bisulfate, respectively, into a reflux reaction device equipped with stirring and oil bath temperature control , add 100mL of petroleum ether, start stirring and adjust the temperature to 100-110°C, and react for 1-1.5h. The conversion rate of phytosterol succinate monoester can reach 98.7% as detected by HPLC. Add 10 mL of distilled water to the reaction solution, extract, collect the organic phase layer and remove the solvent by rotary evaporation to obtain 14.9 g of phytosterol succinate monoester.
[0041] Structural Identification: Phytosterol Succinate FT-IR: 2370-3700cm -1 The broad peak between them is the stretching vibration absorption of hydroxyl-OH in carboxyl-COOH (ν O-H ), 3030cm -1 Absorptio...
Embodiment 2
[0056] Embodiment 2 Phytostanol tea polyphenol adipate diester
[0057] 12.5g of phytostanol, 21.9g of adipic acid, and 1.0g of ionic liquid 1-propylsulfonic acid-3-methylimidazolium p-toluenesulfonate were respectively added to a reflux reaction equipped with stirring and oil bath temperature control. device, add 200mL of tert-butanol, start stirring and adjust the temperature to 80°C-85°C, and react for 2.5h-3h. Heating was stopped and 100 μL was sampled for HPLC analysis. The conversion rate of the intermediate product phytostanol adipate monoester can reach 95.3%.
[0058] After sampling, add 41.2g of hydrophilic modifier tea polyphenol, add 0g of ionic liquid 1-propylsulfonic acid-3-methylimidazolium p-toluenesulfonate, adjust the temperature to 80°C-85°C, and continue the reaction for 5h-6h. Heating was stopped and 100 μL was sampled for HPLC analysis. The conversion rate of the final product phytostanol tea polyphenol adipate diester can reach 92.9%.
[0059] After ...
Embodiment 3
[0060] Embodiment 3 Phytosterol maltitol suberate diester
[0061] Add 12.4g phytosterols, 7.8g suberic acid, and 0.36g ionic liquid 1-butylsulfonic acid-3-methylimidazolium trifluoromethanesulfonate, respectively, into the reflux reaction device equipped with stirring and oil bath temperature control , add 100mL of tert-amyl alcohol, start stirring and adjust the temperature to 100°C-105°C, and react for 1h-1.5h. Heating was stopped and 100 μL was sampled for HPLC analysis. The conversion rate of the intermediate product phytosterol suberate can reach 97.1%.
[0062] After sampling, add 20.6g of hydrophilic modifier maltitol, and add 0.24g of ionic liquid 1-butylsulfonic acid-3-methylimidazole trifluoromethanesulfonate, adjust the temperature to 100℃~105℃, and continue the reaction for 2h~ 3h. Heating was stopped and 100 μL was sampled for HPLC analysis. The conversion rate of the final product phytosterol maltitol suberate can reach 98.2%.
[0063] After stopping the he...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com