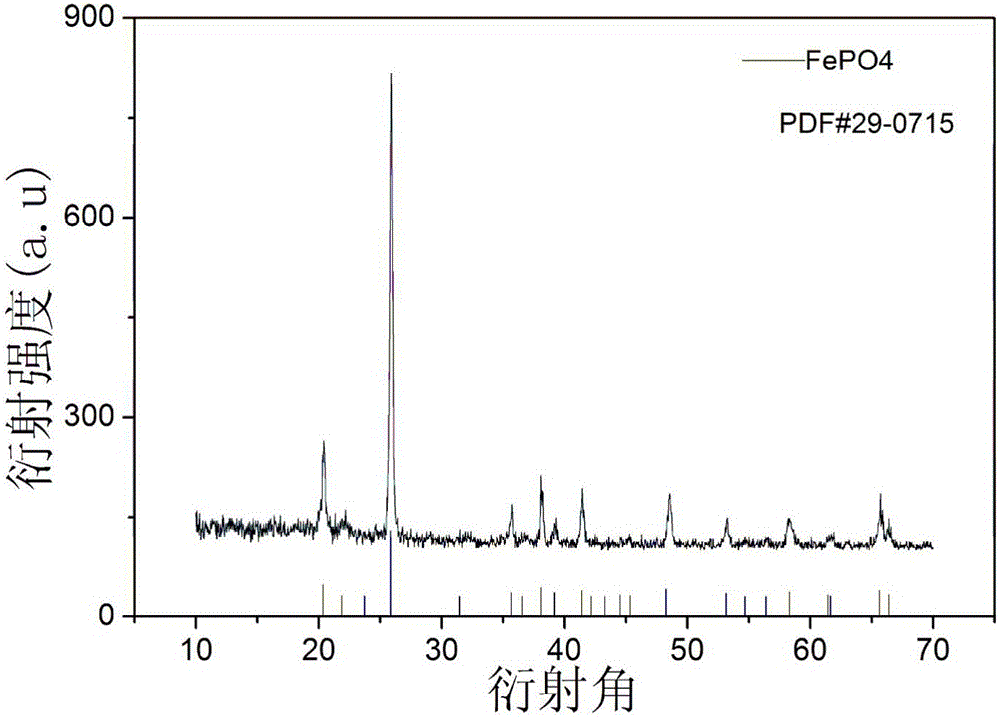
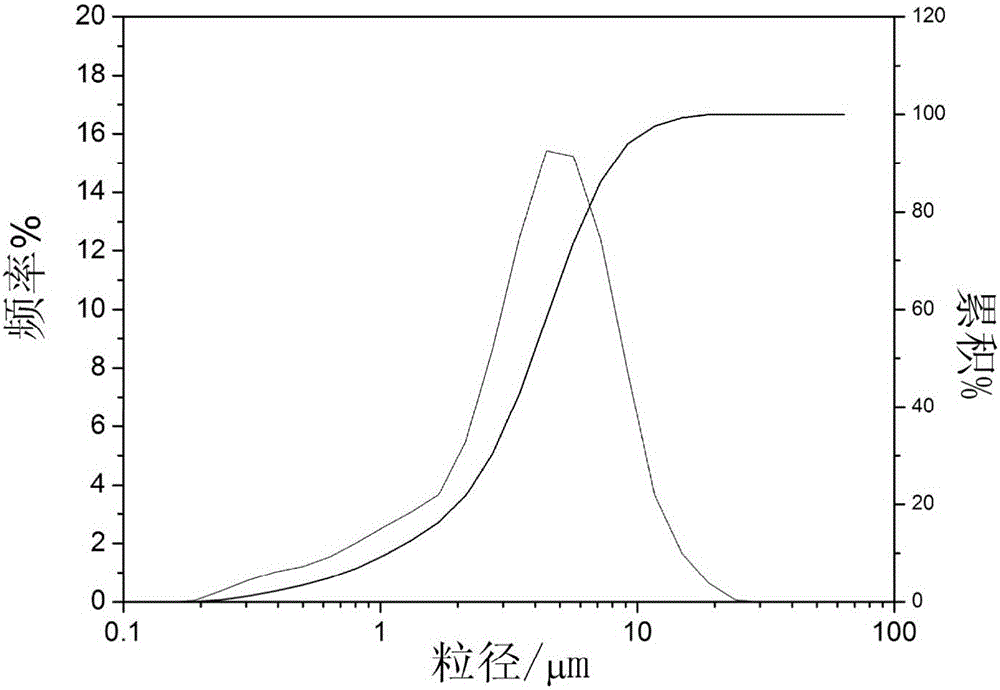
Preparation method of anhydrous ferric pyrophosphate
A technology of ferric orthophosphate and phosphoric acid, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as low purity of ferric phosphate, complex preparation process, and difficult component control, achieving remarkable technological progress and production process Simple, pure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Anhydrous ferric orthophosphate (FePO 4 ) preparation method, specifically comprises the following steps:
[0054] (1) The phosphoric acid of 110 weight parts is added into the deionized water of 300 weight parts to be mixed with phosphoric acid solution, control solution temperature 50 o c. Add 56 parts by weight of 300-mesh reduced iron powder under stirring, and react for 3 hours to obtain a precursor solution containing a phosphorus source and an iron source;
[0055] (2) Add the precursor solution obtained in step (1) into a nano sand mill, and ball mill for 2 hours to obtain a suspension with a D50 of 321 nm in the particle size distribution. The obtained ball mill suspension was spray-dried at 170° C. to obtain spherical precursor powder.
[0056] (3) Finally, after calcining the obtained spherical precursor powder in an air atmosphere at 600° C. for 8 hours, the anhydrous iron orthophosphate powder material is obtained.
[0057]The anhydrous ferric orthophos...
Embodiment 2
[0061] Anhydrous ferric orthophosphate (FePO 4 ) preparation method, specifically comprises the following steps:
[0062] (1) Add 115 parts by weight of phosphoric acid into 300 parts by weight of deionized water to prepare a phosphoric acid solution, and control the temperature of the solution to 65 o c. Add 56 parts by weight of 300-mesh reduced iron powder under stirring, and react for 3 hours to obtain a precursor solution containing a phosphorus source and an iron source;
[0063] (2) Add the precursor solution obtained in step (1) into a nano sand mill, and ball mill for 2 hours to obtain a suspension with a D50 of 258nm in the particle size distribution. The obtained ball mill suspension was spray-dried at 170° C. to obtain spherical precursor powder.
[0064] (3) Finally, after calcining the obtained spherical precursor powder in an air atmosphere at 700° C. for 8 hours, the anhydrous iron orthophosphate powder material is obtained.
[0065] The anhydrous ferric or...
Embodiment 3
[0069] Anhydrous ferric orthophosphate (FePO 4 ) preparation method, specifically comprises the following steps:
[0070] (1) The phosphoric acid of 120 weight parts is added into the deionized water of 300 weight parts and is mixed with phosphoric acid solution, controls solution temperature 80 o c. Add 56 parts by weight of 300-mesh reduced iron powder under stirring, and react for 3 hours to obtain a precursor solution containing a phosphorus source and an iron source;
[0071] (2) Add the precursor solution obtained in step (1) into a nano sand mill, and ball mill for 2 hours to obtain a suspension with a D50 of 217nm in the particle size distribution. The obtained ball mill suspension was spray-dried at 170° C. to obtain spherical precursor powder.
[0072] (3) Finally, after calcining the obtained spherical precursor powder in an air atmosphere at 800° C. for 8 hours, the anhydrous iron orthophosphate powder material is obtained.
[0073] The anhydrous iron orthophos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Median particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com