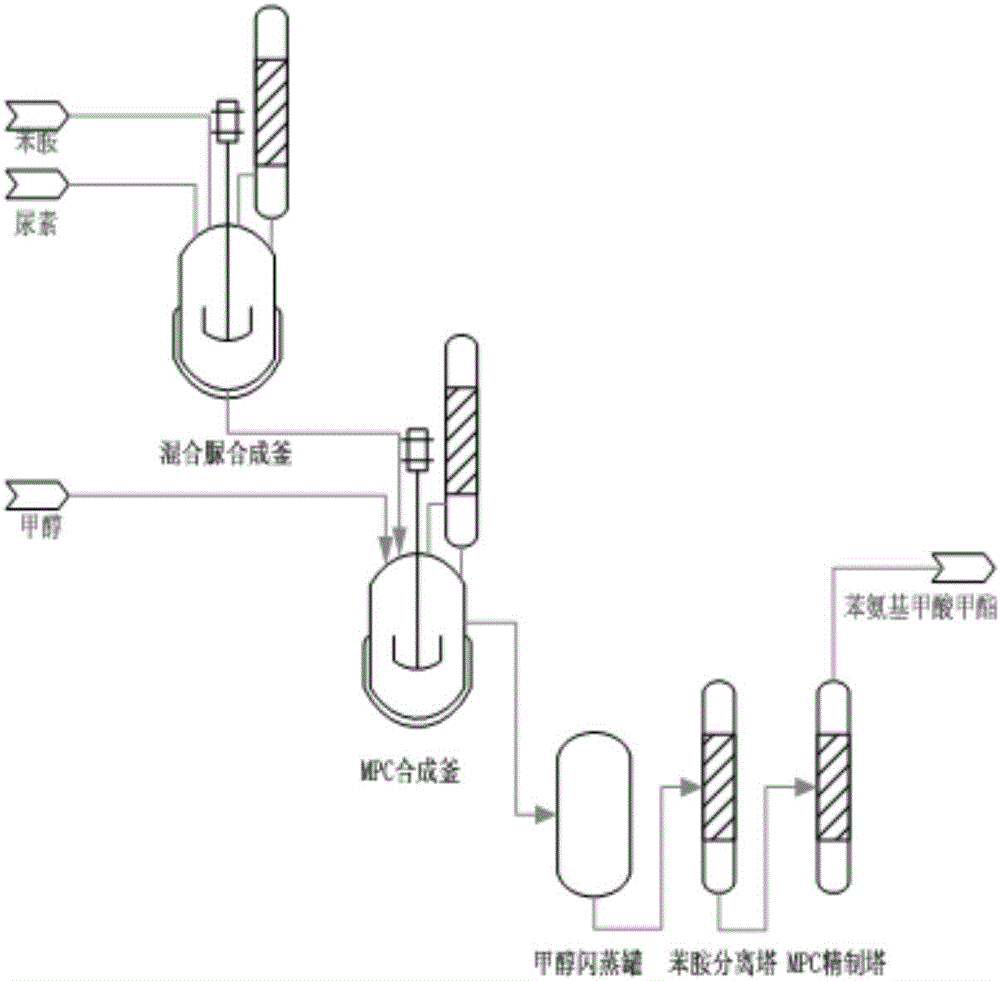
Method for directly synthesizing phenyl carbamate with aniline and urea
A technology of methyl phenylcarbamate and urea, which is applied in the field of synthesis of methyl phenylcarbamate, can solve the problems of low degree of automation, high energy consumption, high cost, etc., achieve simplified operation process, overcome product yield, and be conducive to industrialization The effect of promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 42.4g of urea and 157.6g of aniline were added to a 1L high-pressure reaction and separation device for reaction. After the reaction, 138.7g of reaction product A was collected, and the reaction product A was analyzed by high performance liquid phase to obtain the mass percentage of each component. Phenylurea is 22.7%, diphenylurea is 71.8%, aniline is 5.1%, aniline oxide and ammonium carbonate are less than 0.1%; weigh 90g of methanol and add it to the above reaction product A and mix well, then add 300g of chlorobenzene , seal the reaction device, and pass nitrogen gas with a flow rate of 200ml / min into it, and control the pressure of the reaction device to 2.3MPa, and heat to 175°C. Stay at 175°C for 40 minutes, stop heating, cool down to normal temperature naturally, finally turn on the device, collect 268.5g of reaction product B, conduct high performance liquid phase analysis on the reaction product B, and obtain the contents of each component in the product are mo...
Embodiment 2
[0029] 60.0g of urea and 204.6g of aniline were added to a 1L high-pressure reaction separation device for reaction. After the reaction, 189.5g of reaction product A was collected. The reaction product A was analyzed by high performance liquid phase, and the mass percentage of monophenylurea was 28.7%. , the mass percentage of diphenylurea is 66.8%, and the mass percentage of aniline is 4.3%. Weigh 63.8g methyl alcohol and join in the above-mentioned reaction product A and mix homogeneously, then add o-dichlorobenzene 300g, seal reaction device, feed flow velocity wherein into the nitrogen that is 10ml / min, and the pressure of control reaction device is 2.5MPa, and heated to 175°C. Stay at 175°C for 40 minutes, stop heating, cool down to normal temperature naturally, finally open the device, collect 548.3g of reaction product B, and perform high performance liquid phase analysis on the reaction product B, in which monophenylurea, diphenylurea Urea accounts for 0.21%, methyl p...
Embodiment 3
[0032]50.0 g of urea and 186.0 g of aniline were added to a 1L high-pressure reaction separation device for reaction. After the reaction, 174.2 g of reaction product A was collected, and the reaction product A was analyzed by high performance liquid phase to obtain the mass percentage of each component. Weigh 40.0g of methanol and add it to the above-mentioned reaction product A and mix evenly, then add 200g of diethyl phthalate, seal the reaction device, and feed nitrogen gas with a flow rate of 600ml / min into it, and control the pressure of the reaction device It is 4MPa and heated to 200°C. Stay at 200°C for 20 minutes, stop heating, cool down to normal temperature naturally, finally turn on the device, collect 408.9g of reaction product B, conduct high performance liquid phase analysis on the reaction product B to obtain the content of each component in the product, of which monophenylurea Undetectable, diphenylurea accounted for 0.23%, methyl phenylcarbamate accounted for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com