Kit for detecting clostridium difficile and application thereof
A detection kit, a technology for Clostridium difficile, which can be applied to measurement devices, instruments, scientific instruments, etc., can solve the problems of poor detection effect and high false positive rate, achieve efficient and rapid detection, improve sensitivity, and have good application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 The present invention is used for detecting the kit of Clostridium difficile
[0025] 1, the composition of kit of the present invention
[0026] (1) diluent of the present invention:
[0027] Formula 1: PBS (PH7.4), 1% NP-40, 0.5% TritonX-100, 1% BSA, 0.05% Tween20
[0028] Formula 2: PBS (PH7.4), 1% NP-40, 1% TritonX-100, 1% BSA, 0.05% Tween20
[0029] Formula 3: PBS (PH7.4), 1% NP-40, 2% TritonX-100, 1% BSA, 0.05% Tween20
[0030] PBS: Phosphate Buffered Saline
[0031] (2) blocking solution of the present invention
[0032] Formula 1: PBS, 2% BSA, 1% trehalose, 1% FBS
[0033] Formula 2: PBS, 2% BSA, 2% trehalose, 2% FBS
[0034] Formula 3: PBS, 2% BSA, 3% trehalose, 3% FBS
[0035] Formula 4: PBS, 2% BSA, 5% trehalose, 5% FBS
[0036] (3) Other components: ELISA plate coated with specific antibody, HRP enzyme-labeled secondary antibody, positive control substance (GLDH recombinant antigen, ToxinA / B recombinant antigen), washing solution, chromo...
experiment example 1
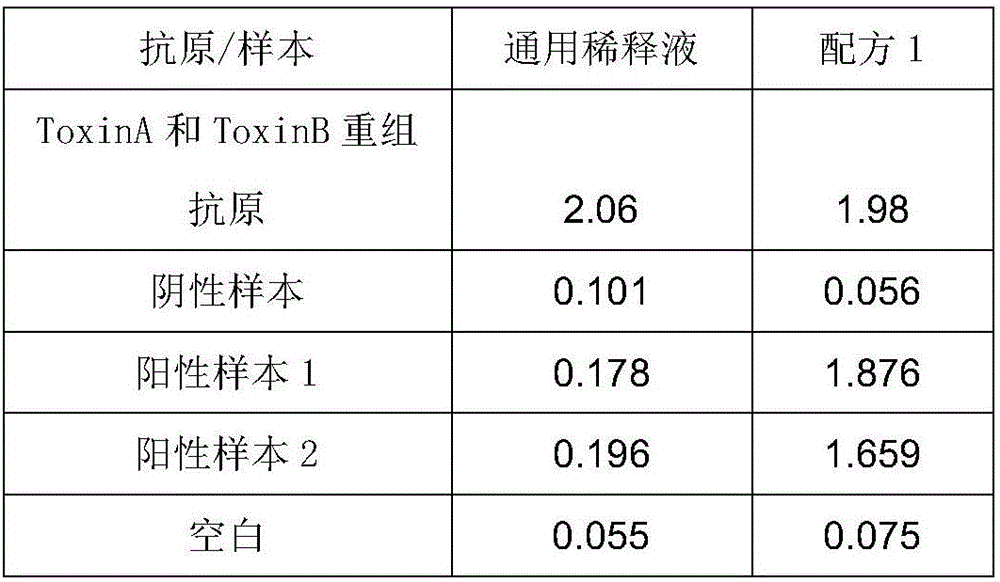
[0044] Comparison of Experimental Example 1 and Existing Universal Diluent
[0045] 1. Experimental materials
[0046] The kit of the present invention: the kit of Example 1 of the present invention is used, wherein the blocking solution is Formula 2, and the diluent of Formula 1 to Formula 10 is used as the diluent.
[0047] Formula 1: PBS (PH7.4), 1% NP-40, 0.5% TritonX-100, 1% BSA, 0.05% Tween20
[0048] Formula 2: PBS (PH7.4), 1% NP-40, 1% TritonX-100, 1% BSA, 0.05% Tween20
[0049] Formula 3: PBS (PH7.4), 1% NP-40, 2% TritonX-100, 1% BSA, 0.05% Tween20
[0050] Formula 4: PBS (PH7.4), 0.1% NP-40, 1% BSA, 0.05% Tween20
[0051]Formula 5: PBS (PH7.4), 1% NP-40, 1% BSA, 0.05% Tween20
[0052] Formula 6: PBS (PH7.4), 5% NP-40, 1% SDS, 1% BSA, 0.05% Tween20
[0053] Formula 7: PBS (PH7.4), 1% NP-40, 1% SDS, 1% BSA, 0.05% Tween20
[0054] Formula 8: PBS (PH7.4), 1% NP-40, 2% SDS, 1% BSA, 0.05% Tween20
[0055] Formula 9: PBS (PH7.4), 1% NP-40, 1% SDS, 0.5% TritonX-100, 1...
experiment example 2
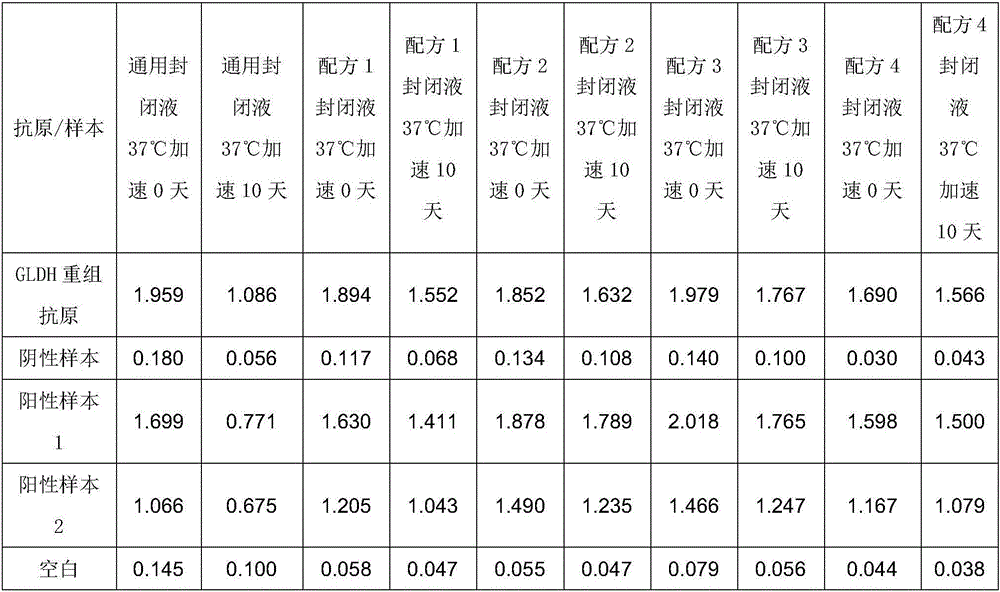
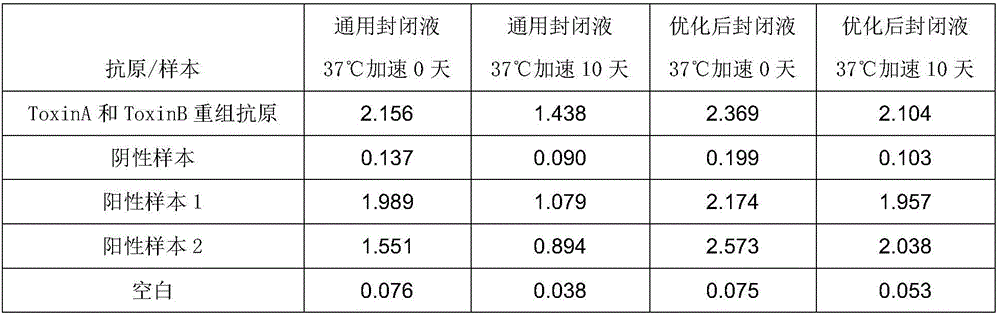
[0072] Comparison of Experimental Example 2 and the Existing Universal Blocking Solution
[0073] 1. Experimental materials
[0074] The kit of the present invention: the kit of Example 1 of the present invention is used, wherein the blocking liquid is formula 1 to formula 4, and the diluent is formula 1 respectively.
[0075] Control kit: except that the diluent is a universal blocking solution, other components are the same as the kit of the present invention, and the universal blocking solution is: PBS (PH7.4), 1% BSA, 0.05% Tween20.
[0076] 2. Experimental method
[0077] Get GLDH recombinant antigen, positive sample 1,2 (toxigenic Clostridium difficile culture supernatant), negative sample (common E. detection.
[0078] The detection method is as follows:
[0079] Use different blocking solutions to block the ELISA plates coated with specific antibodies. After the blocking solutions of each formula are sealed, part of them is stored at 4°C, and the other part is subj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com