Method for preparing chromatographic stationary phase by using divinyl sulphone as template
A technology of divinyl sulfone and chromatographic stationary phase, which is applied in the field of chromatographic stationary phase, can solve the problems of affecting chromatographic separation performance, non-uniform bonded stationary phase surface, lengthy preparation route, etc., and achieve simple synthesis route and convenient and easy-to-obtain raw materials , the effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
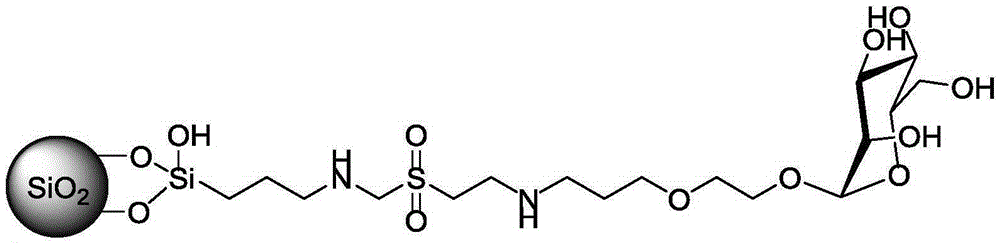
[0028] Under nitrogen protection, take 1.66 g of aminopropyltrimethoxysilane reagent, dissolve it in 20 mL of anhydrous tetrahydrofuran solution, stir it electromagnetically, add 1.027 g of divinyl sulfone reagent, adjust the pH value to 7.5 with triethylamine, stir at room temperature, and Phase chromatographic detection yields a divinylsulfone-bonded silane reagent. Under nitrogen protection, dissolve the dried silica gel (2.5g) in 30mL of anhydrous toluene, add divinylsulfone-bonded silane reagent, and dropwise add pyridine (6mmol, 480uL), heat to reflux, react for 12h, stop the reaction , cooled and suction-filtered, washed with anhydrous toluene, tetrahydrofuran, methanol, water, and methanol successively, dried and solidified at 80°C for 6 hours, and obtained divinyl sulfone-activated silica gel, named as SNDVS material.
[0029] Weigh 2.5g of the above dried SNDVS material, under nitrogen protection, dissolve in 10mL of anhydrous N,N-dimethylamide (DMF), add glucosamine...
Embodiment 2
[0031] With the operation process of embodiment 1, difference is:
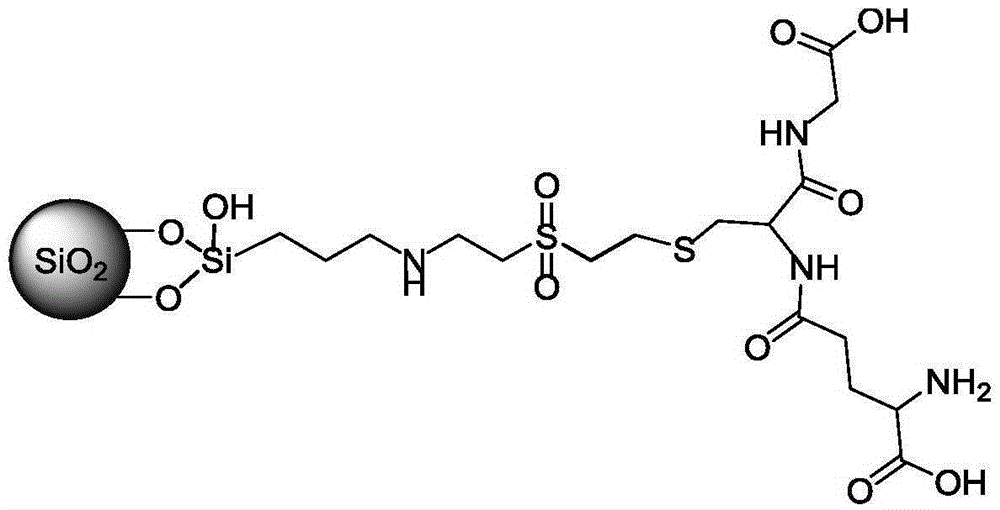
[0032] Weigh 2.5g of the above dried SDVS material, under nitrogen protection, dissolve in 10mL of anhydrous methanol solvent, add glutathione (6mmol, 1.84g), triethylamine (6mmol, 840μL) and stir at room temperature for 10h, stop the reaction, reduce Filter under pressure, wash with 30mL methanol, 20mL water, and 20mL methanol successively, dry and solidify at 60°C for 6h to obtain SNDVS-GSH and store at room temperature.
[0033]
Embodiment 3
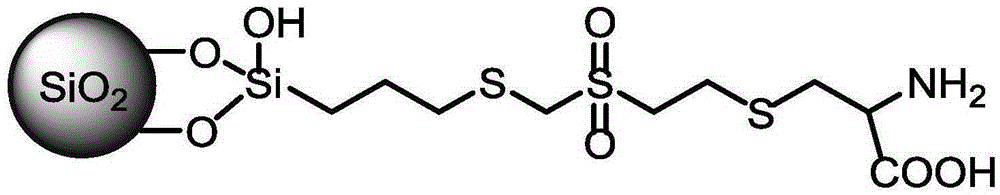
[0035] Under nitrogen protection, take 1.05 g of mercaptopropyltriethoxysilane reagent, dissolve it in 20 mL of anhydrous tetrahydrofuran solution, stir it electromagnetically, add 1.21 g of divinyl sulfone reagent, adjust the pH value to 7.5 with triethylamine, and stir at room temperature. Liquid chromatographic detection obtained divinyl sulfone modified mercaptosilane reagent. Under nitrogen protection, dissolve the dried silica gel (2.5g) in 30mL of anhydrous toluene, add divinylsulfone-bonded silane reagent, and dropwise add pyridine (6mmol, 480uL), heat to reflux, react for 12h, stop the reaction , cooled and suction-filtered, washed with anhydrous toluene, tetrahydrofuran, methanol, water, and methanol successively, dried and solidified at 80°C for 6 hours, and obtained divinyl sulfone-activated silica gel, which was named SSDVS material.
[0036] Weigh 2.5g of the above-mentioned dry SSDVS material, under nitrogen protection, dissolve in 10mL of anhydrous N, N-dimethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com