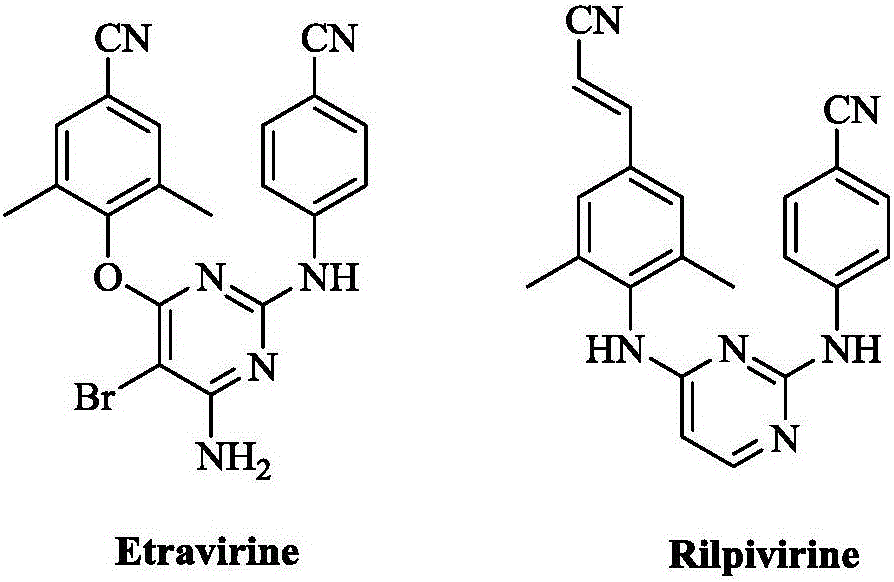
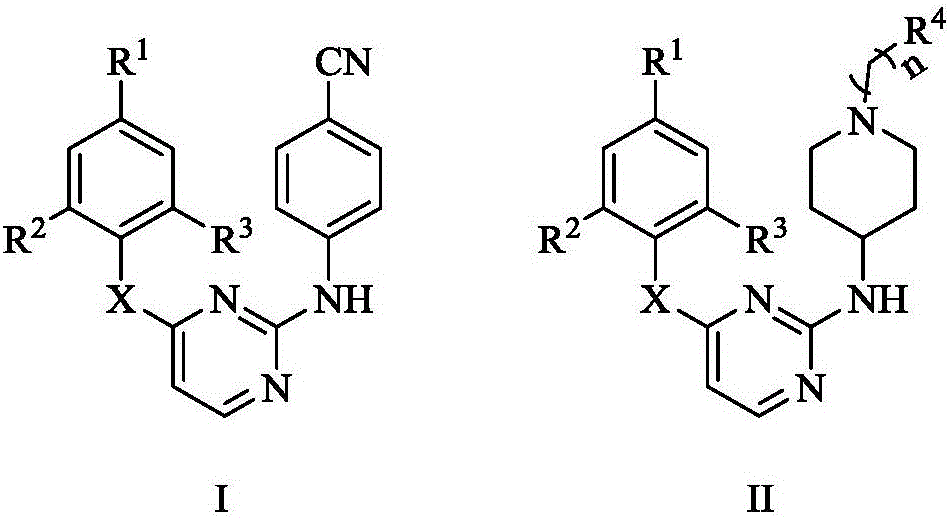
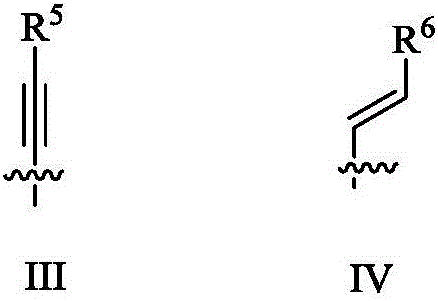Substituted diarylpyrimidine derivative as well as preparation method and application thereof
A derivative, dimethylformamide technology, applied in the field of medicine, can solve the problems of low bioavailability, poor membrane permeability, cross-resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the preparation of intermediate 4-((4-chloropyrimidin-2-yl) amino) benzonitrile
[0062] Preparation of 4-((4-oxo-1,6-dihydropyrimidin-2-yl)amino)benzonitrile (2)
[0063] Weigh 2-(methylthio)pyrimidin-4(3H)-one (3g, 21mmol) and 4-aminobenzonitrile (2.99g, 25mmol) in a 50mL round-bottom flask, nitrogen protection, and slowly heat up to 180°C, Reaction 8h. After the reaction was cooled, 20 mL of acetonitrile was added for sonication, filtered, the filter cake was washed with acetonitrile, no 4-aminobenzonitrile residue was detected by TLC, and the filter cake was dried to obtain a pale yellow solid which was 4-((4-oxo-1,6 -Dihydropyrimidin-2-yl)amino)benzonitrile, yield 73.6%, ESI-MS: m / z 213.3[M+H] + ,C 11 h 8 N 4 O(212.12).
[0064] Preparation of intermediate 4-((4-chloropyrimidin-2-yl)amino)benzonitrile
[0065] Accurately weigh 4-((4-oxo-1,6-dihydropyrimidin-2-yl)amino)benzonitrile (0.80g, 3.8mmol), add 5mL phosphorus oxychloride, stir and reflu...
Embodiment 2
[0066] Embodiment 2: the preparation of target compound
[0067] Preparation of 4-((3-aminophenyl)ethynyl)-2,6-dimethylphenol
[0068] Weigh 3-aminophenylacetylene (0.1g, 0.85mmol), 4-iodo-2,6-dimethylphenol (0.254g, 1.0mmol), bistriphenylphosphine dichloride palladium (0.0359g, 0.51mmol ), cuprous iodide (0.0195g, 1.0mmol), triethylamine (0.06g, 0.6mol) were dissolved in anhydrous tetrahydrofuran, under nitrogen protection, reacted at 60°C for 10h, after the TLC detection reaction was completed, the reaction solution was diatom After soil filtration, evaporate to dryness under reduced pressure, add 30 mL ethyl acetate to the residual substrate, wash with saturated saline solution 3 times, 10 mL each time, separate the organic layer, dry over anhydrous sodium sulfate, filter and concentrate. The intermediate 4-((3-aminophenyl)ethynyl)-2,6-dimethylphenol was separated by flash column chromatography.
[0069] Preparation of 4-((4-(4-((3-aminophenyl)ethynyl)-2,6-dimethylphenoxy...
Embodiment 3
[0106] Embodiment 3: the preparation of target compound
[0107] Preparation of 2,6-dimethyl-4-styrylphenol
[0108] Weigh styrene (0.177g, 1.5mmol), 4-iodo-2,6-dimethylphenol (0.248g, 1.0mmol), palladium acetate (0.0224g, 0.1mmol), Tol 3 P (0.091g, 0.3mmol), sodium ethoxide (0.184g, 2.3mmol) in a double-neck flask, add N,N-dimethylacetamide dropwise with a constant pressure dropping funnel under nitrogen protection, and heat up to 60°C , reacted for 10 h, after the reaction, the solvent was evaporated to dryness, and the crude product of intermediate 5 was obtained by flash column separation.
[0109] (E)-4-((4-(2,6-Dimethyl-4-styrylphenoxy)pyrimidin-2-yl)amino)benzonitrile
[0110] The operation is the same as the preparation of IB-1-1, except that 2,6-dimethyl-4-styrylphenol is used.
[0111]
[0112] The product is white crystals, yield: 38%, melting point: 222-224°C.
[0113] 1 H NMR (400MHz, DMSO-d 6 )δ10.15(s,1H,NH),8.47(d,J=5.6Hz,1H,C6-pyrimidine-H),7.69-7.59(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com