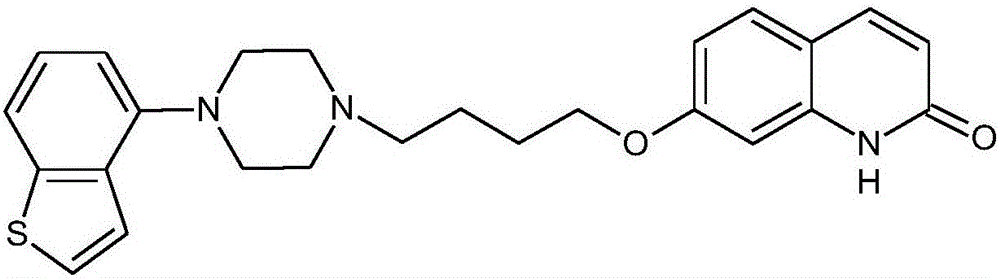
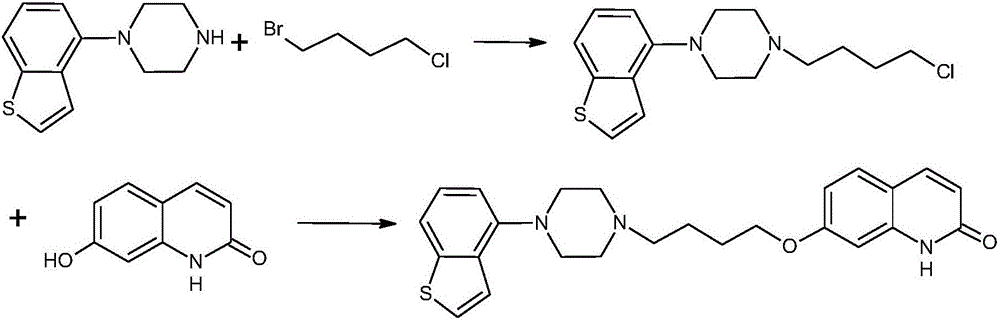
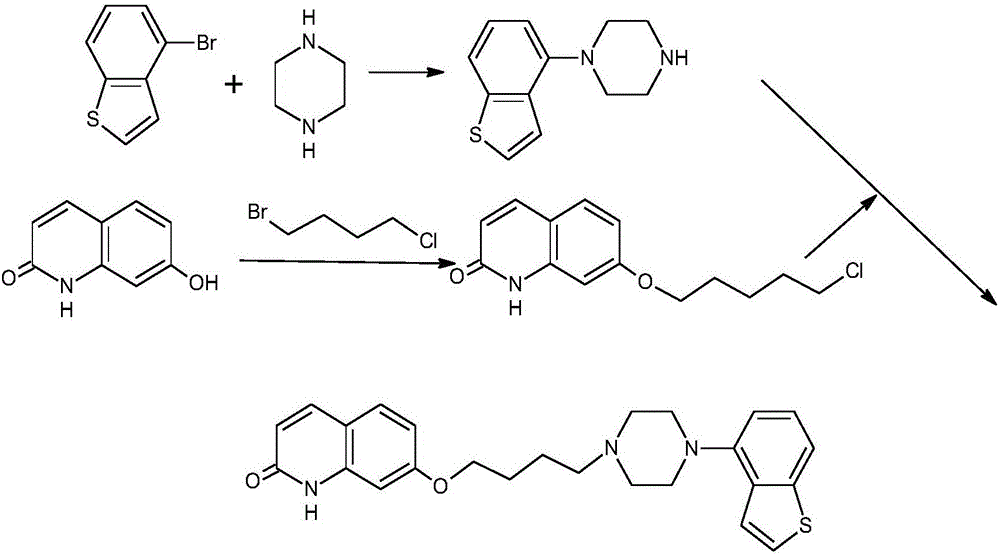
Preparation method for synthesizing brexpiprazole
A technology of ebiprazole and compounds, applied in a new field of preparation, can solve the problems of increased risk of industrial production, difficulty in large-scale production, long reaction steps, etc., and achieve low implementation cost, strong industrial application value, and high product quality Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (a) Put 80g (0.446mol) of 4-nitrobenzene[b]thiophene into a 500mL autoclave, 4g (0.05g / g) of 5% palladium carbon, and 320mL of methanol. 3 times, fill with hydrogen to a pressure of 0.3 MPa, stir, heat up to 50°C and react for 6 hours, keep warm until no hydrogen is absorbed in the kettle, keep warm for 30 minutes, cool down to room temperature, take out the reaction solution, and filter under nitrogen protection. The filtrate was concentrated under reduced pressure until the remaining volume was 150 mL to obtain compound (II) with a weight of 60 g and a yield of 90%.
[0068] (b) Under nitrogen protection, 81.6g (0.5mol) of 7-hydroxyl-1H-quinolin-2-one, 103g (0.6mol, 1.2ep) of bromochlorobutane, 103.5g (0.75 ep) of potassium carbonate were put into the flask mol, 1.5ep), acetone 816mL, stirred and heated to 60°C, and reacted for 8 hours. After the reaction is completed, cool down to 20°C, filter, concentrate the filtrate to recover a part of acetone, add water dropwis...
Embodiment 2
[0074] (a) drop into 4-nitrobenzene [b] thiophene 80g (0.446mol) in 500ml autoclave, Raney nickel 8g (0.1g / g), ethanol 400ml, feed intake complete, nitrogen replacement 3 times, then hydrogen replacement 3 times Once, fill in hydrogen gas to a pressure of 1MPa, stir, raise the temperature to 80°C and react for 5 hours, keep the temperature until the hydrogen is no longer absorbed in the kettle, keep the temperature for 30 minutes, cool down to room temperature, take out the reaction solution, and suction filter under the protection of nitrogen. Concentrate under reduced pressure until the remaining volume is 200ml, stir and crystallize to obtain compound (II) with a weight of 58g and a yield of 87%.
[0075](b) under nitrogen protection, put into the flask 80.6g (0.5mol) of 7-hydroxy-1H-quinolin-2-one, 86g (0.5mol, 1ep) of bromochlorobutane, 53g (0.5mol, 1ep) of sodium carbonate ), 484 ml of N,N-dimethylformamide, and stirred at 100°C for 6 hours. After the reaction is comple...
Embodiment 3
[0081] (a) 80g (0.446mol) of 4-nitrobenzene[b]thiophene, 2.4g (0.03g / g) of 10% palladium on carbon, 500ml of ethanol were put into a 500ml autoclave, the feeding was completed, nitrogen was replaced 3 times, and then hydrogen Replaced 3 times, filled with hydrogen to a pressure of 0.2 MPa, stirred, heated at 20 °C for 8 hours, kept the reaction at a temperature until no more hydrogen was absorbed in the kettle, kept the reaction for another 30 minutes, cooled to room temperature, took out the reaction solution, and suction filtered under nitrogen protection , the filtrate was concentrated under reduced pressure until the remaining volume was 200ml, and the mixture was stirred and crystallized to obtain compound (II) with a weight of 59g and a yield of 88%.
[0082] (b) under nitrogen protection, put into the flask 80.6g (0.5mol) of 7-hydroxy-1H-quinolin-2-one, 103g (0.6mol, 1.5ep) of bromochlorobutane, 106g (1mol, 1.5ep) of sodium bicarbonate 2ep), 645 ml of N,N-dimethylacetam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com