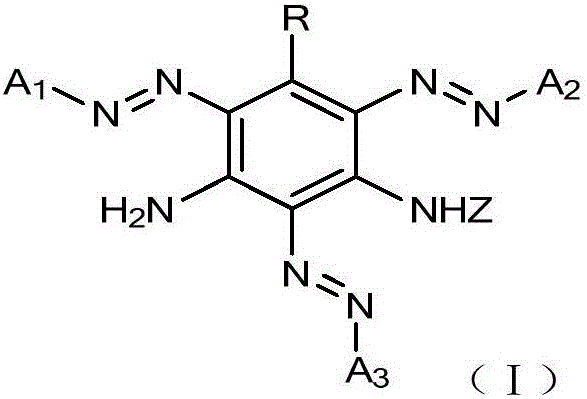
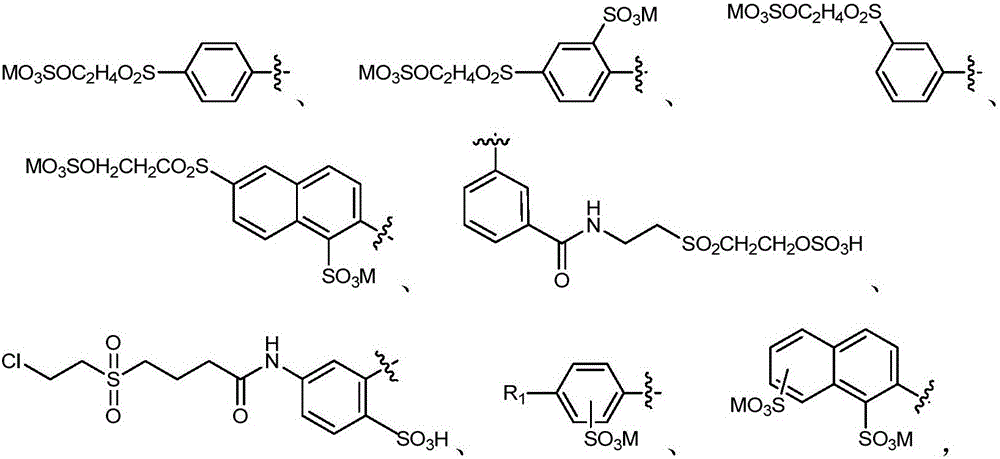
Active orange dye and preparation and application thereof
A reactive orange and dye technology, applied in reactive dyes, azo dyes, organic dyes, etc., can solve the problems of high water consumption, high energy consumption, and low actual utilization rate of dyeing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
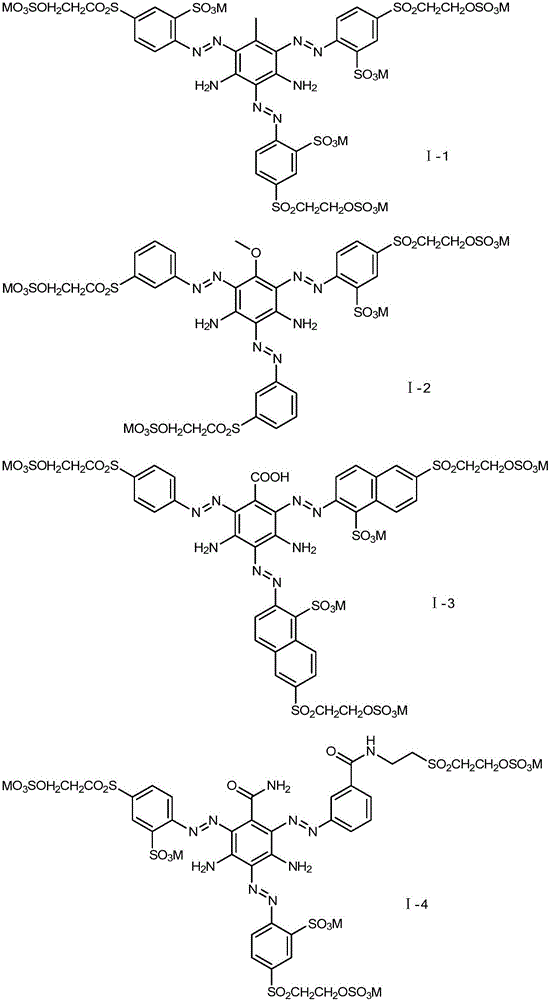
[0050] Embodiment 1: the preparation of formula (I-1) compound
[0051] (1) Diazo, coupling reaction
[0052] In a 250ml beaker, add 20 parts (parts by mass) of ice, 24.3 parts of 30% hydrochloric acid, 36.1 parts of sulfonated para-ester (diazo component 1), ice grinding and beating for 30min, slowly add 6.97 parts of sodium nitrite to generate diazonium salt After the sodium nitrite is completely added, stir for 1 hour, and use sulfamic acid to eliminate the slight excess of sodium nitrite; slowly add the diazonium salt dropwise to 12.2 parts of 3,5-diaminotoluene (coupling component), drop Use baking soda to adjust pH=3-5 while adding, continue to react for 2 hours after adding, the diazonium salt will disappear completely, while keeping the system temperature at 0-10°C.
[0053]
[0054] (2) Primary coupling component and secondary coupling of diazonium salt
[0055] Add 40 parts of ice, 48.6 parts of 30% hydrochloric acid, 72.2 parts of sulfonated para-ester (diazo c...
Embodiment 2
[0057] Embodiment 2: the preparation of formula (I-2) compound
[0058] (1) Diazo, coupling reaction
[0059] In a 250ml beaker, add 20 parts of ice, 24.3 parts of 30% hydrochloric acid, 36.1 parts of sulfonated para-ester, ice mill and beat for 30 minutes, slowly add 6.97 parts of sodium nitrite to form diazonium salt, and stir for 1 hour after the sodium nitrite is completely added , use sulfamic acid to eliminate the slight excess of sodium nitrite; slowly add the diazonium salt to 13.8 parts of 3,5‐diaminoanisole, and adjust the pH to 3‐5 with sodium bicarbonate while adding. Afterwards, the reaction was continued for 2 hours while maintaining the system temperature at 0-10°C, and the diazonium salt disappeared completely.
[0060]
[0061] (2) Primary coupling component and secondary coupling of diazonium salt
[0062] In a 250ml beaker, add 40 parts of ice, 48.6 parts of 30% hydrochloric acid, 56.2 parts of meta-ester, ice grinding and beating for 30 minutes, slowly...
Embodiment 3
[0064] Embodiment 3: the preparation of formula (I-3) compound
[0065] (1) Diazo, coupling reaction
[0066] In a 250ml beaker, add 20 parts of ice, 24.3 parts of 30% hydrochloric acid, 28.1 parts of para-ester, ice grinding and beating for 30 minutes, slowly add 6.97 parts of sodium nitrite to generate diazonium salt, stir for 1 hour after the sodium nitrite is completely added, and use Sulfamic acid eliminates slightly excess sodium nitrite; slowly add the diazonium salt dropwise to 15.2 parts of 3,5-diaminobenzoic acid, adjust the pH to 3-5 with baking soda while adding, and continue the reaction after the addition is complete 2h, while keeping the system temperature at 0-10°C, the diazonium salt completely disappeared.
[0067]
[0068] (2) Primary coupling component and secondary coupling of diazonium salt
[0069] In a 250ml beaker, add 40 parts of ice, 48.6 parts of 30% hydrochloric acid, 82 parts of 6-hydroxyethyl sulfone sulfate-2-naphthylamine-1-sulfonic acid, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com