Method for preparing alpha-fluoro-beta-ethynyl ketone compound containing two chiral centers
A technology of ethynyl ketone and chiral center, which is applied in the field of preparing α-fluoro-β-ethynyl ketone compounds containing two chiral centers, achieving the effect of simple synthesis, good reactivity and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
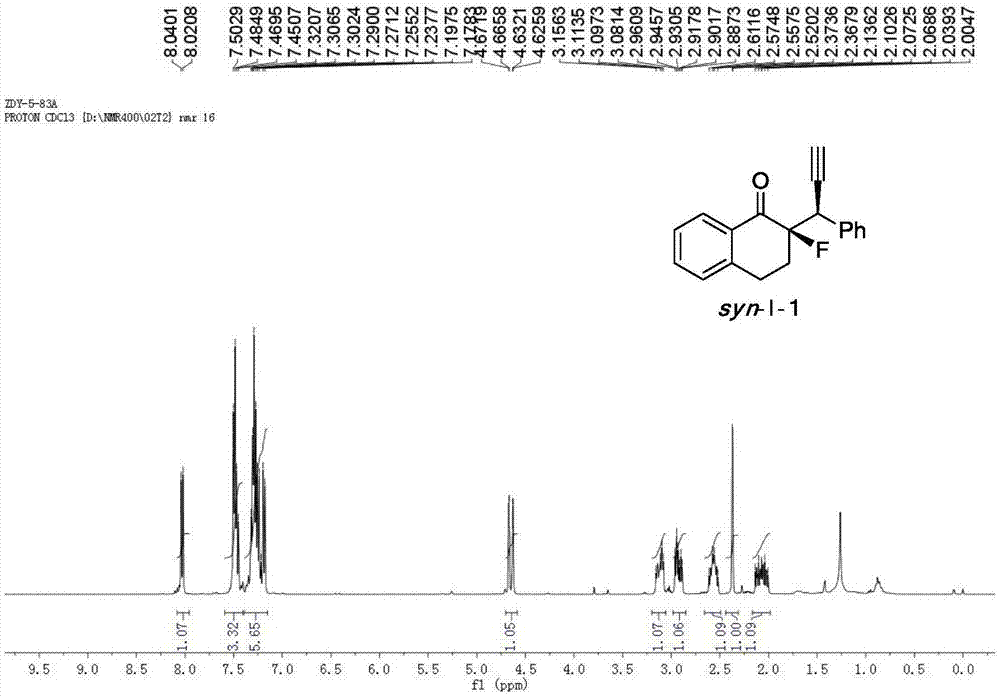
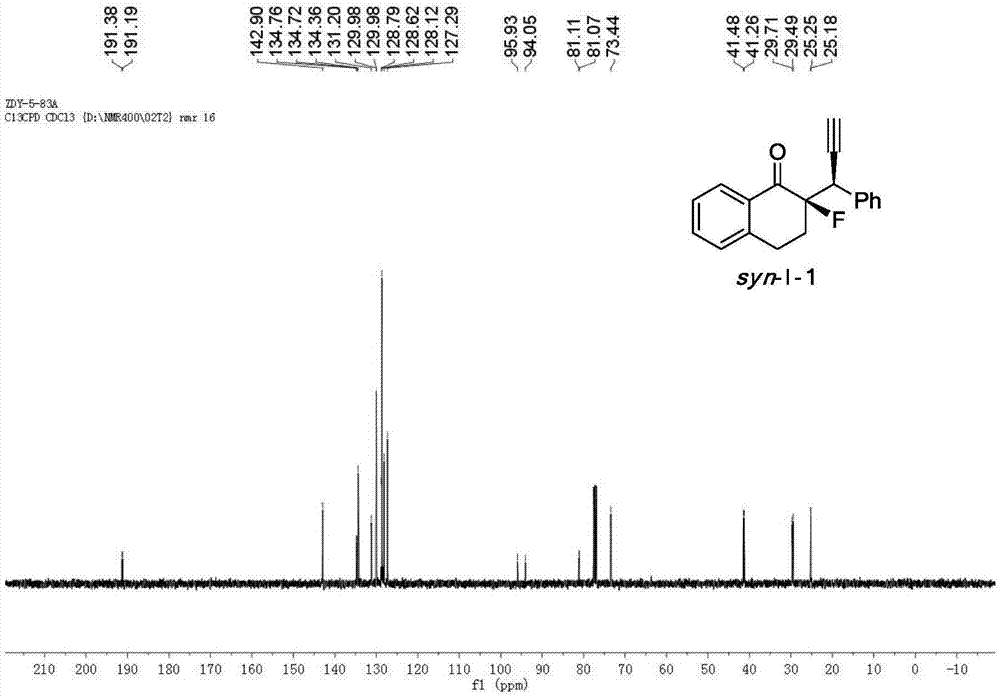
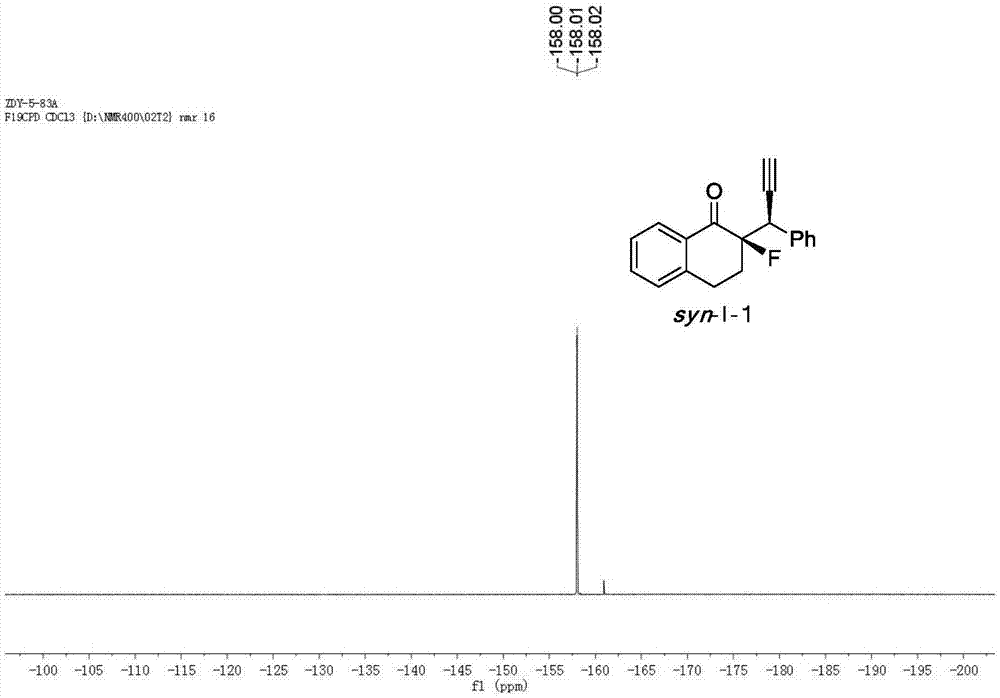
[0048] Cu(CH 3 EN) 4 BF 4 Complexation with L-1-1 acts as a catalyst to catalyze the reaction to generate chiral α-fluoro-β-ethynyl ketone product I-1.
[0049] Add the metal precursor Cu(CH 3 EN) 4 BF 4 (0.015mmol, 5mol%) and chiral ligand L-1-1 (0.0165mmol, 5.5mol%) were added to 1.0ml of anhydrous methanol under nitrogen protection, and stirred at room temperature for 1 hour. Fluorinated enol silyl ether II-1 (0.30mmol, 1.0equiv), propargyl alcohol ester III-1 (0.75mmol, 2.5equiv) and N,N-diisopropylethylamine (0.75mmol, 2.5equiv) Dissolve in 2.0 ml of anhydrous methanol, and then add the solution to the above-mentioned stirred catalyst solution under the protection of nitrogen, and react with stirring at -20° C. for 12 h. After the reaction was completed, the reaction was rotary evaporated under reduced pressure, and the residue was flushed into a short column of silica gel. After the rotary evaporation, the residue was sent to NMR to determine the dr value, and then...
Embodiment 2
[0055] L-2-1 acts as a ligand to generate chiral α-fluoro-β-ethynyl ketone product I-1
[0056] The ligand L-1-1 in Example 1 is replaced by the ligand L-2-1, and the rest are the same as in Example 1. The reaction gave compound I-1 in 70% yield, 75 / 25dr(syn / anti), 98%ee(syn), 99%ee(anti).
[0057] The structural formula of L-2-1 is as follows:
[0058]
Embodiment 3
[0060] L-2-2 reacts as a ligand to generate the product α-fluoro-β-ethynyl ketone product I-1
[0061] The ligand L-2-1 in Example 2 is replaced by the ligand L-2-2, and the rest are the same as in Example 1. Compound Ⅰ-1 was obtained in 60% yield, 67 / 33dr(syn / anti), 88%ee(syn), 94%ee(anti).
[0062] The structural formula of L-2-2 is as follows:
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com