Preparation and application of cytokine mutant fusion antibody
A technology of fusion antibodies and mutants, applied in the field of bioengineering, can solve the problems of limited function and low affinity, and achieve strong inhibitory effect and growth inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Construction of Fully Human Anti-VEGFR2 Fusion Antibody Gene
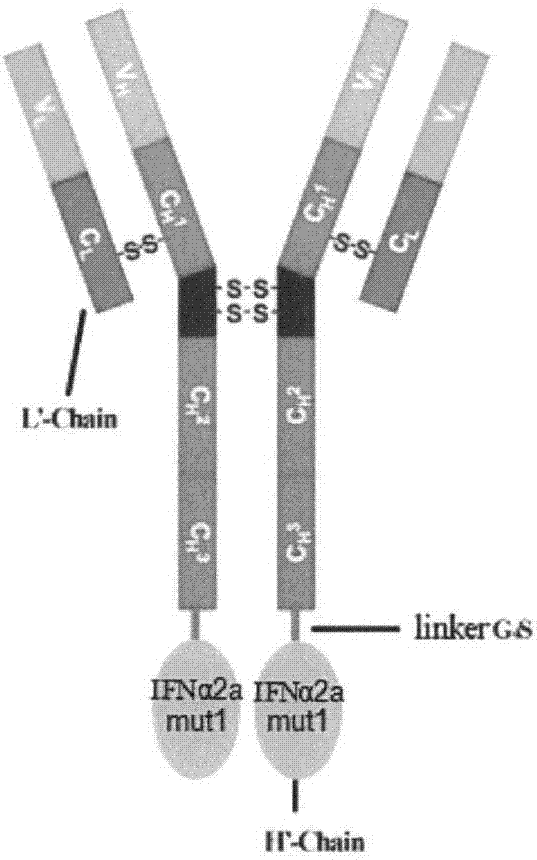
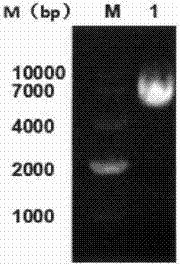
[0034] Our research group has successfully obtained the recombinant plasmid of anti-VEGFR2 fusion antibody JZA01. JZA01 is at the C-terminus of antibody Fc segment through G 4S Linker is conjugated to the fusion antibody of IFNα. In its heavy chain expression plasmid, the two ends of the IFNα expression part contain restriction sites BamHI and PmeI. Based on this, first obtain the sequence expressing IFNα by double enzyme digestion of the plasmid, then use the obtained IFNα as a template, design mutation primers, use overlap PCR to obtain IFNαmut, and then replace the JZA01-H chain with the restriction sites at both ends. The part of wild-type IFNα was obtained by expression vector JZA02-H chain. A large amount of the obtained mutant expression vector and the common light chain L chain were extracted and prepared to be transfected into CHO cells.
Embodiment 2
[0035] Example 2 Expression, purification and SDS identification of anti-VEGFR2 fusion antibody
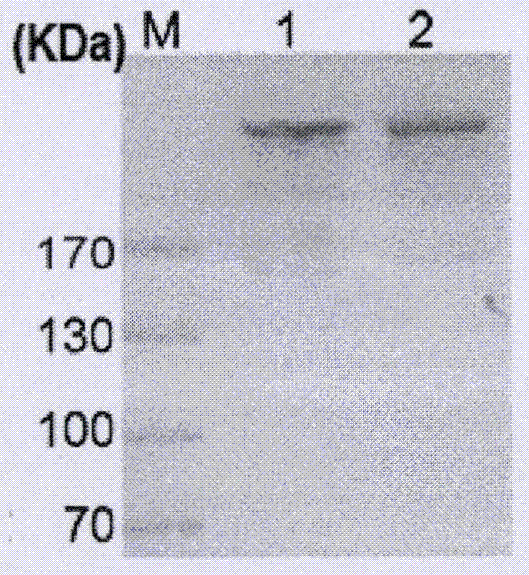
[0036] The light and heavy chains were transiently transfected into CHO cells, and stable and high-yielding monoclonal cell lines were selected by pressure selection and two limited dilution methods. The stable high-yielding cell line was expanded and cultured, the supernatant was collected, centrifuged at 6000 rpm, 4°C for 30 min, filtered with a 0.22 μm filter membrane, purified with a Protein A affinity chromatography column, and the eluent was used to elute the protein. The purified JZA02 and JZA01 were mixed with non-reducing SDS-PAGE loading buffer at a ratio of 4:1 (20μl:5μl) in an Eppendorf tube, placed in a water bath at 100°C for 5 minutes, then centrifuged at 3000rpm for 5 minutes, and the protein samples were prepared. stand-by. Configure 10% SDS-PAGE gel for electrophoresis, stop electrophoresis after 80mA constant current for 30min, 120min constant current for 30min...
Embodiment 3
[0037] Western Blot identification of embodiment 3 anti-VEGFR2 fusion antibody
[0038] Purified JZA01, JZA02, and JZA00 were subjected to SDS-PAGE electrophoresis, 80V constant voltage for 30min, 120V constant voltage for 30min, and transfer for 2h. The protein was transferred to PVDF membrane (purchased from Millipore). (TBS containing 5% skimmed milk) at room temperature for 2 hours; dilute the primary antibody with 5% MTBS at 1:2000 (A&B picture is HRP-coupled goat anti-human Fc antibody without secondary antibody; C&D picture is rabbit antibody Human κ chain antibody; E&F picture is mouse anti-human IFNα antibody) (purchased from abcam), incubated at room temperature for 1.5h, washed three times with TBST, washed three times with TBS, each 10min; diluted 1:5000 with 5% MTBS Secondary antibody (C&D picture is HRP-conjugated goat anti-rabbit secondary antibody; E&F picture is goat anti-mouse secondary antibody) (purchased from Lianke Biology), incubated at room temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com