PLK1 inhibitor, and preparation method and application thereof
A cutting agent and condensation reaction technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problem of difficulty in penetrating cell membranes, decreased binding force, and transmembrane transport of oligonucleotides and small RNA molecules Difficult to solve and other problems, to achieve the effect of overcoming the problem of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
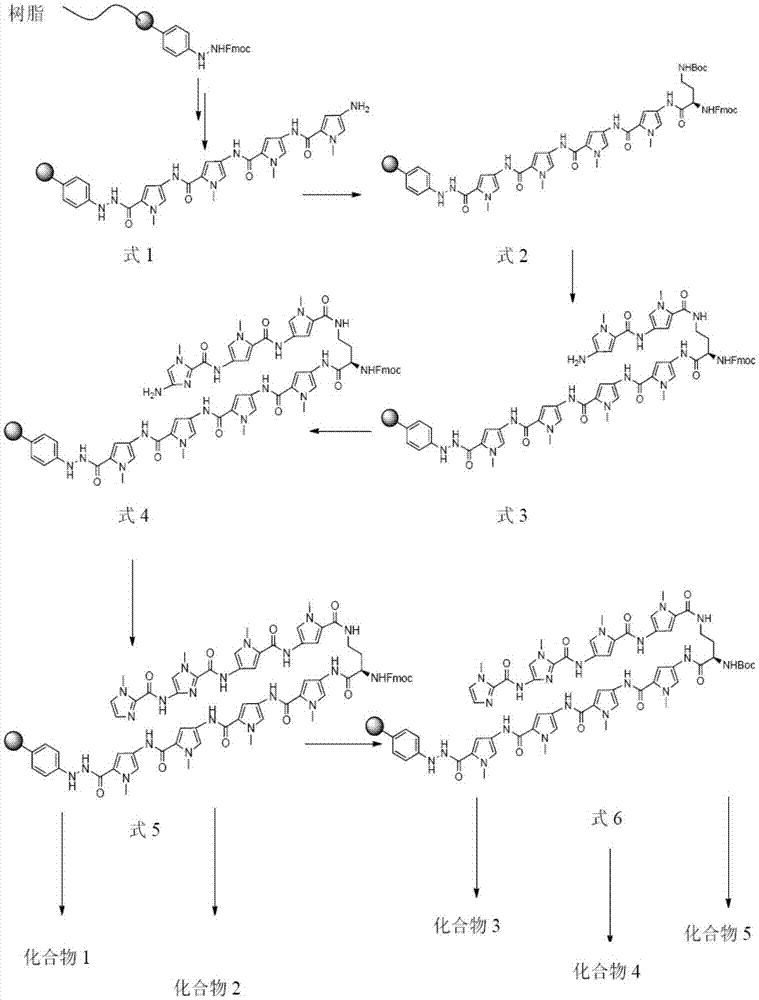
[0094] Example 1 Preparation of Compound 1
[0095] (a) Resin swelling: Add 400mg Fmoc protected phenylhydrazine resin (0.66mmol / g, 0.264mmol) and 3mL CH to a 10mL solid phase reactor 2 Cl 2 , Swell the resin for 30 minutes, and remove CH 2 Cl 2 ,spare;
[0096] (b) Removal of Fmoc protecting group: Add 3 mL of 20% piperidine / DMF solution to the swelled resin in step (a), N 2 Mix well by bubbling. After 10 minutes, remove the solvent and add 3 mL of 20% piperidine / DMF solution, N 2 Mix well by bubbling. After 10 minutes, wash the resin with DMF (4×3mL), and then wash the resin with 3mL of anhydrous DMF for later use;
[0097] (c) Amino acid condensation: 4-tert-butoxycarbonylamino-1-methyl-1H-pyrrole-2-carboxylic acid (254mg, 1.056mmol) and triphosgene (BTC, 128mg, 0.433mmol) were dissolved in 2mL of anhydrous THF, slowly dropwise add collidine (collidine, 488μL, 3.696mmol) to the solution, the reaction immediately produces a large amount of white precipitate, after adding the reacti...
Embodiment 2
[0113] Example 2 Preparation of Compound 2
[0114] The peptide of formula (5) supported on phenylhydrazine resin was prepared according to the same steps of Example 1, and the step (b) in Example 1 was used to remove the peptide of formula (5) supported on phenylhydrazine resin. Fmoc protecting group in the above peptide;
[0115] The Hester acid derivative Ht-1 (539 mg, 1.056 mmol) and PyBOP (550 mg, 1.056 mmol) were dissolved in 3 mL of anhydrous DMF, DIEA (350 μL, 2.112 mmol) was added, and the reaction was carried out for 5 min. The reaction solution was transferred to the In addition to Fmoc, the peptide represented by formula (5) supported on phenylhydrazine resin, N 2 Bubbling and mixing, condensation reaction for 1h, aspirate the reaction liquid, wash the resin with DMF (4×3mL); take out the resin, add 1mL DMF, 200μL dimethylaminopropylamine and 10mg Cu(OAc) 2 , Shake the reaction at room temperature for 12h, filter out the resin, and use 20mL CH 2 Cl 2 Wash the resin; con...
Embodiment 3
[0119] Example 3 Preparation of Compound 3
[0120] The peptide of formula (5) supported on phenylhydrazine resin was prepared according to the same steps of Example 1, and the step (b) in Example 1 was used to remove the peptide of formula (5) supported on phenylhydrazine resin. Fmoc protecting group in the above peptide;
[0121] Will Boc 2 O (243 μL, 1.056 mmol) was dissolved in 3 mL anhydrous DMF, DIEA (350 μL, 2.112 mmol) was added, and the reaction solution was transferred to the Fmoc-removed peptide represented by formula (5) supported on phenylhydrazine resin, N 2 Bubbling and mixing, condensation reaction for 20min. The reaction solution was removed, and the resin was washed with DMF (4×3 mL) to obtain the Boc-protected peptide represented by formula (6) supported on the phenylhydrazine resin;
[0122]
[0123] Take out the resin obtained above, add 1mL DMF, 200μL N,N-bis(3-aminopropyl)methylamine, shake the reaction at 90℃ for 1h, cool to room temperature, filter out the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com