Injectable hydrogel, and preparation method
A technology for injecting water and gel, which is used in pharmaceutical formulations, medical preparations with inactive ingredients, aerosol delivery, etc. Good compatibility, good biodegradability, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] In the present invention, the preparation method of the arginine-modified chitosan preferably comprises the following steps:
[0062] a) mixing chitosan, arginine derivatives and an activator for amidation reaction to obtain a compound shown in formula (II);
[0063]
[0064] The arginine derivative has a structure shown in formula (III):
[0065]
[0066] b) deprotecting the compound represented by formula (II) to obtain arginine-modified chitosan.
[0067] In the present invention, chitosan, arginine derivatives and activators are firstly mixed for amidation reaction to obtain the compound represented by the formula (II). In the present invention, described chitosan has structure shown in formula (IV):
[0068]
[0069] In the present invention, the molecular weight of the chitosan is preferably 2000Da to 500000Da, more preferably 8000Da to 300000Da, more preferably 10000Da to 200000Da; Commercially available products well known to those skilled in the art...
Embodiment 1
[0112] (1) Weigh 2g chitosan and mix it with 200mL hydrochloric acid solution to obtain the first mixed solution; then weigh 0.2g arginine derivative, 0.2g EDC, 0.1g NHS in a round bottom flask, add 20mL DMF to dissolve it , to obtain a second mixed solution; adding the second mixed solution to the first mixed solution, stirring and reacting at 30° C. for 48 hours, and purifying to obtain a solid powder;
[0113] (2) The solid powder obtained in step (1) is dispersed as the reaction medium with 100mL trifluoroacetic acid, 15mL of hydrogen bromide solution is added, reacted for 6h at 30°C, and purified to obtain arginine-modified chitosan, the yield is 80%.
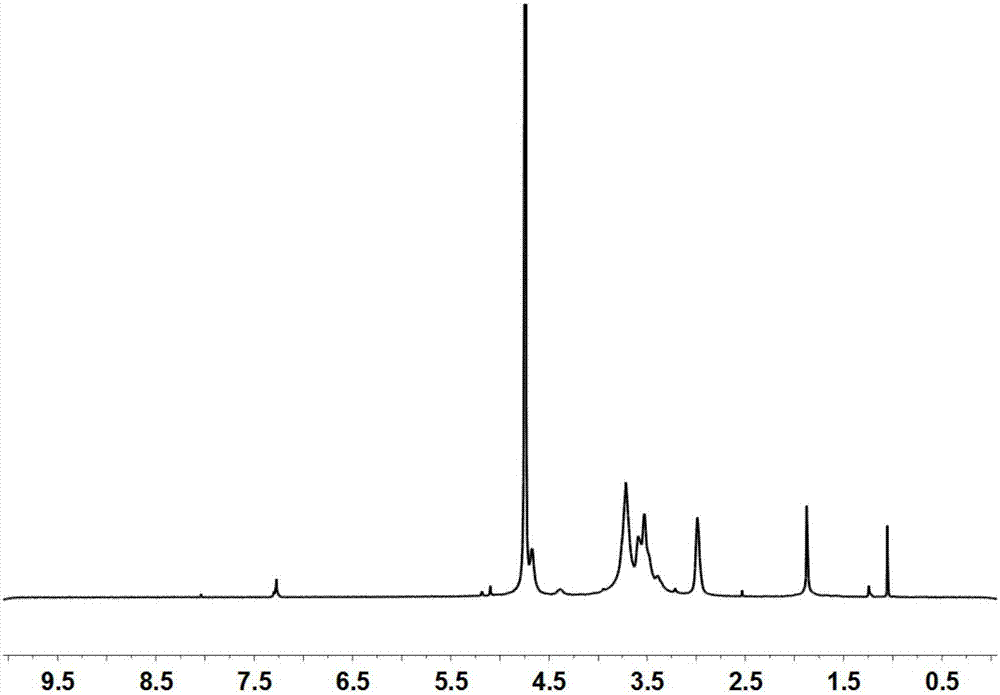
[0114] Carry out nuclear magnetic resonance analysis to the solid powder that step (1) obtains, such as figure 1 shown. The results showed that arginine was successfully attached to chitosan, and the substitution degree of arginine was 3%.
Embodiment 2
[0116] (1) Mix 2g chitosan with 200mL hydrochloric acid solution to obtain the first mixed solution; then weigh 0.4g arginine derivative, 0.4g EDC, 0.2g NHS in a round bottom flask, add 20mL DMF to dissolve it , to obtain a second mixed solution; adding the second mixed solution to the first mixed solution, stirring and reacting at 30° C. for 48 hours, and purifying to obtain a solid powder;
[0117] (2) The solid powder obtained in step (1) is dispersed as the reaction medium with 100mL trifluoroacetic acid, 15mL of hydrogen bromide solution is added, reacted for 6h at 30°C, and purified to obtain arginine-modified chitosan, the yield is 85%.
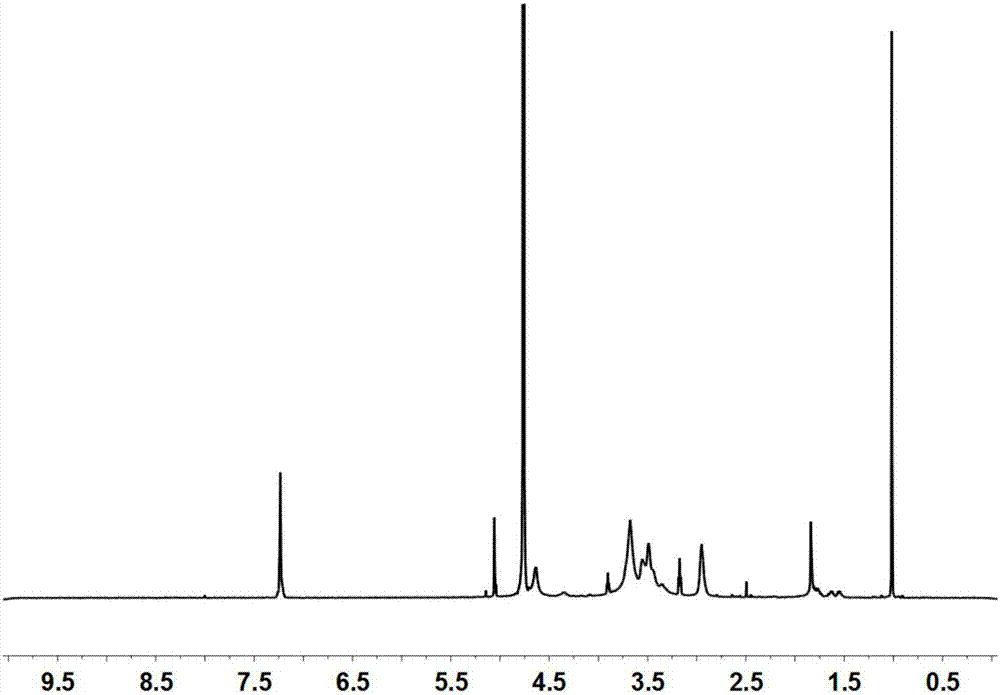
[0118] The solid powder obtained in step (1) was subjected to nuclear magnetic resonance analysis, and the results showed that arginine was successfully connected to chitosan, and the substitution degree of arginine was 6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com