Highly-stable urea reagent and detection method
A detection method and reagent technology, applied in the field of urea detection reagents, can solve problems such as poor reagent stability, and achieve the effects of good stability, improved stability, and enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Urea detection reagents, including reagent R1 and reagent R2:
[0056] 1) The composition of its R1 is:
[0057] Imidazole-HCL buffer ············································ 100mmol / L,
[0058] BSA ······················································· 1g / L,
[0059] ɑ-KG ······················································ 8mmol / L
[0060] sucrose ······················································ 5g / L,
[0061] Trehalose ···················································· 2g / L,
[0062] polyethylene glycol ·················································· 5g / L,
[0063] xanthan gum ···················································· 2g / L,
[0064] Mannitol ···················································· 4g / L,
[0065] maltose ···················································· 5g / L,
[0066] GLDH ······················································ 2KU / L,
[0067] Triton X-100· ············································· 2mL / L...
Embodiment 2
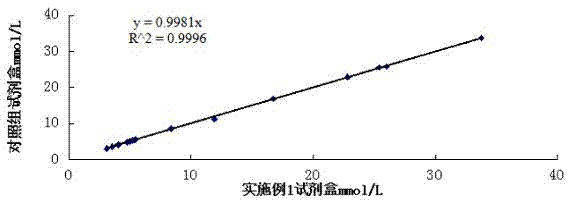
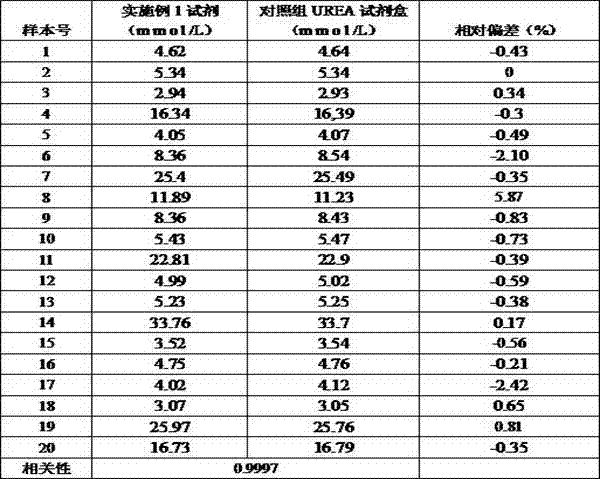
[0089] Correlation experiment: using the formula in Example 1 to prepare reagents, and conducting a control test with the urea kit of a company approved by the State Food and Drug Administration, which is common in the market, and testing 20 clinical serum samples at the same time, the test results are shown in Table 2 . And obtained the correlation curve of the two reagents (such as figure 1 Shown), the test results show that the correlation coefficient of the two kits is 0.9997, indicating that there is a great correlation between the two.
[0090] Table 2 Example 1 reagent and the common and approved urea assay kit comparative detection results in the market
[0091]
Embodiment 3
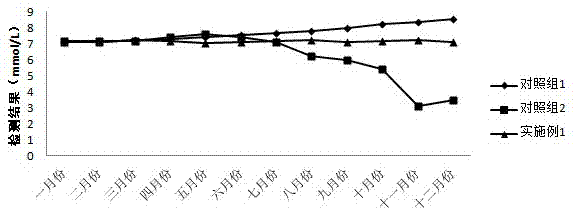
[0093] Contrast test of the stability of the validity period of the reagent: For the reagent in Example 1, 13 groups were evenly distributed, and the reagent volume of each group was 15 mL for R1 and 5 mL for R2; A urea kit from a certain company was used as a control. Place it in a refrigerator at 2-8°C, take out a set of reagents on the same day every month to test the urine quality control product (target value is 7.31mmol / L), the test results are as follows: figure 2 As shown, the reagent of Example 1 is more stable than the common urea assay kits in the market under the storage condition of 2-8°C.
[0094] Through verification, this reagent has a good correlation with similar detection reagents, and the results of clinical test samples are consistent, which can meet the application requirements of the market for products. It is a more stable and good urea detection reagent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com