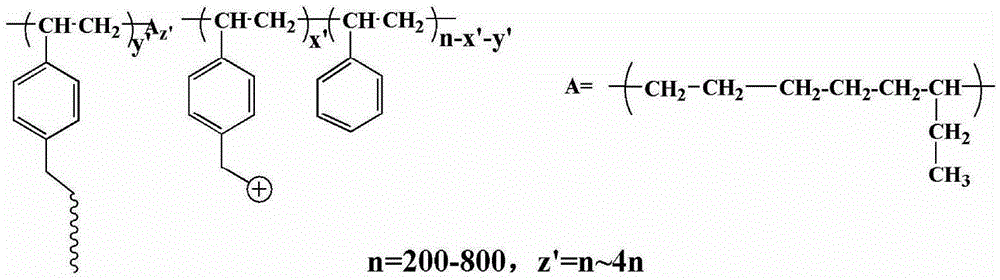Alkaline anion exchange membrane and preparation method thereof
A basic anion, exchange membrane technology, applied in the field of the preparation of anion exchange membranes, can solve the problems of reduced membrane conductivity, reduced membrane, membrane loss of ion-conducting ability, etc., to achieve good mechanical properties, surface uniformity, good chemical stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
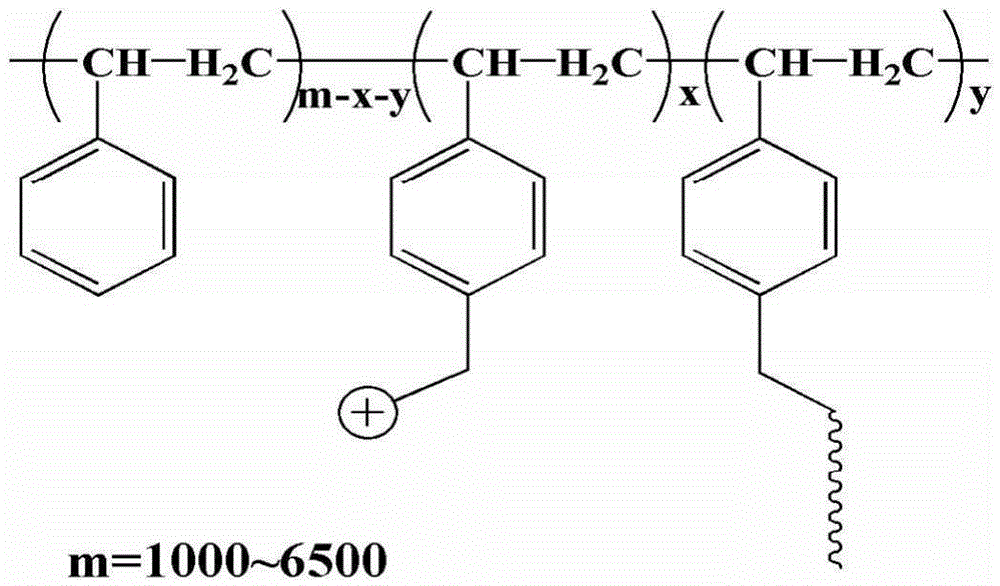
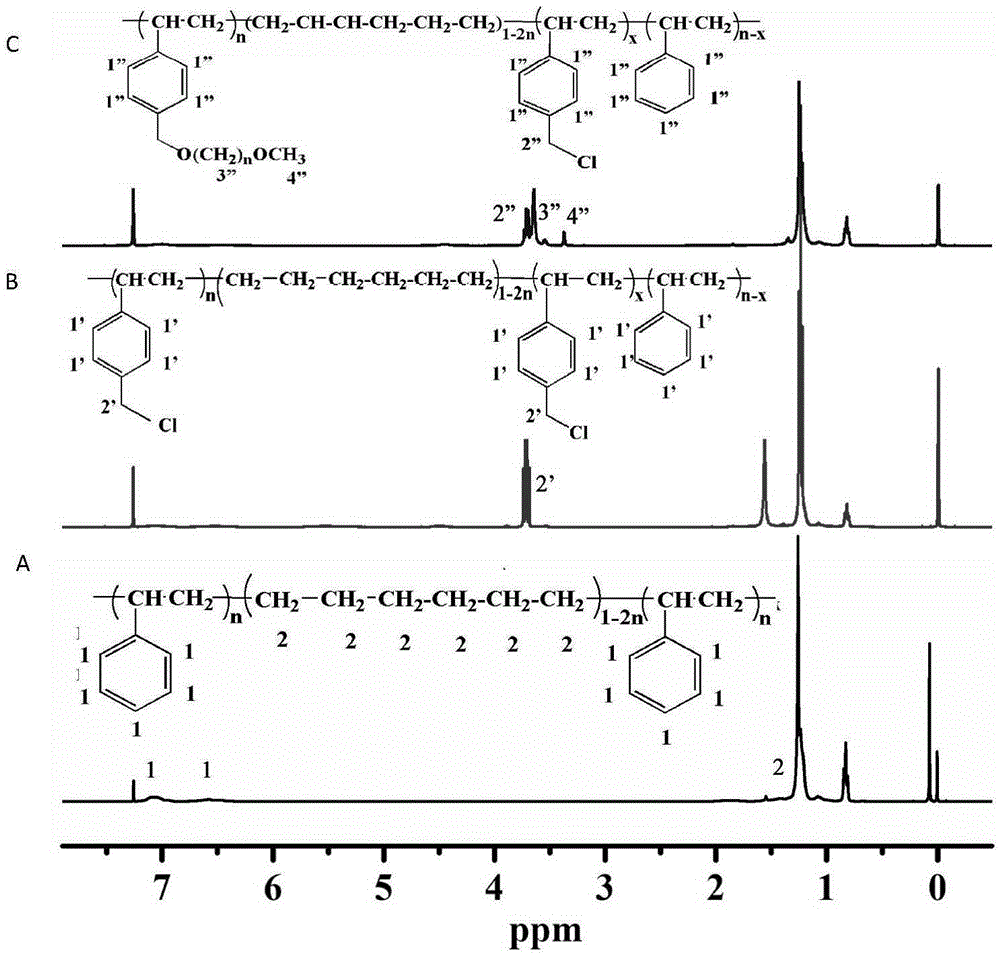
[0061] 2 g of a poly(styrene-ethylene-butylene) block copolymer with a molecular weight of about 70,000 and a styrene content of 30% was dissolved in 30 mL of CCl 4 Add 3.5g of anhydrous tin tetrachloride and 4g of 1,4-dichloromethoxybutane sequentially under the condition of ice-water bath (~5°C), stir for 0.5h under the condition of ice-water bath, and then under the condition of 17°C Under reaction 12h. After the reaction solution returned to room temperature, it was poured into ethanol to precipitate a light yellow solid, which was dissolved in tetrahydrofuran and then precipitated with ethanol. The operation was repeated three times, and then the solid was vacuum-dried at room temperature for 12 hours to obtain chloromethylated poly(styrene - ethylene-butene) block copolymers.
[0062] Dissolve 0.5 g of the above-prepared chloromethylated poly(styrene-ethylene-butylene) block copolymer in 10 mL of tetrahydrofuran, slowly add 25 mg of NaH, and then add 0.25 g of polyethyl...
Embodiment 2
[0075] Dissolve 2 g of poly(styrene-ethylene-butylene) block copolymer with a molecular weight of about 440,000 and 50% styrene content in 120 mL of tetrachloroethane, and add 10 g of ZnCl successively under ice-water bath conditions 2 1. 20 g of chloromethylhexyl ether, stirred for 1 h in an ice-water bath, and then reacted at 20° C. for 4 h. After the reaction solution returned to room temperature, it was poured into ethyl acetate to precipitate a light yellow solid, which was washed three times with ethyl acetate, and then the solid was dried in vacuum at 30°C for 12 hours to obtain chloromethylated poly(styrene-ethylene-butylene ) block copolymers.
[0076] Dissolve 0.3 g of the above-prepared chloromethylated poly(styrene-ethylene-butylene) block copolymer in 18 mL of tetrahydrofuran, slowly add 0.15 g of NaH, and then add 0.1 g of polyethylene glycol monomer with a molecular weight of 1000 Methyl ether was heated and stirred at 30°C for 24 hours. After the reaction solu...
Embodiment 3
[0080] 2 g of polystyrene with a molecular weight of 104,000 was dissolved in 60 mL of 98 wt % concentrated sulfuric acid, 10 g of 1,4-dichloromethoxybutane was added in an ice-water bath, and the reaction was stirred for 24 h in an ice-water bath. The reaction solution was poured into methanol to precipitate a white solid, and the solid was vacuum-dried overnight at 20° C. to obtain chloromethylated polystyrene.
[0081] Dissolve 0.5 g of the above-prepared chloromethylated polystyrene in 5 mL of dimethylacetamide, slowly add 0.1 g of potassium tert-butoxide, then add 2.0 g of polyethylene glycol monomethyl ether with a molecular weight of 350, and Stir the reaction at 20°C for 48 hours, pour the reaction solution into ethanol, and precipitate a slightly yellowish colloidal solid, which is fully washed with ethanol, and dried in a vacuum oven at room temperature for 48 hours. According to the nuclear magnetic spectrum analysis of chloromethylated polystyrene and band hydropho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com