Polysaccharide hydrogel with double responses of pH and oxidation reduction as well as preparation method and application of polysaccharide hydrogel
A dual-response, hydrogel technology, applied in non-active ingredients medical formulations, aerosol delivery, liquid delivery, etc., can solve the uncertainty of synthetic polymer biocompatibility, limit the application of hydrogel, High economic cost, to achieve the effect of good biocompatibility, low price and extensive resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
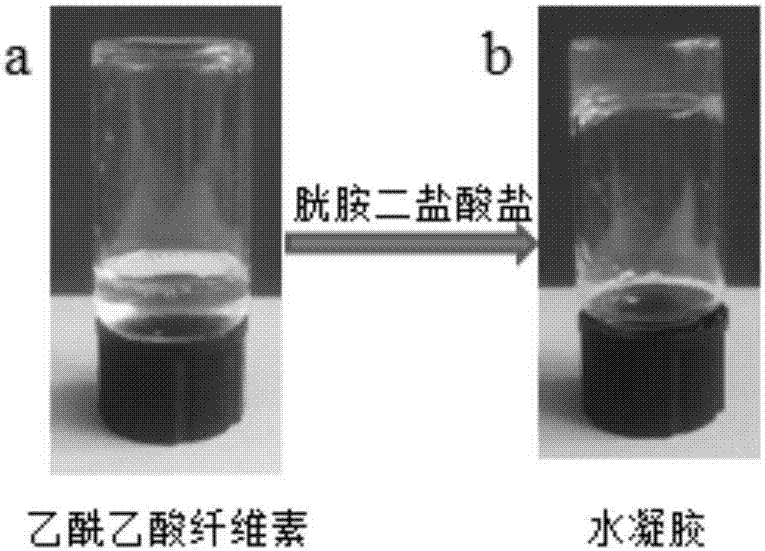
[0029] 1) Deionized water was used to prepare a 2 wt% acetoacetate wood pulp cellulose solution and a 10 wt% cystamine dihydrochloride solution. At room temperature, 0.1965g of 10wt% cystamine dihydrochloride solution was added to 2g of 2wt% cellulose acetoacetate solution, after mixing uniformly on a vortex shaker, let stand for 40min to form a gel ( figure 1 ).
Embodiment 2
[0031] 1) The preparation process and raw materials of multiple responsive polysaccharide hydrogels are the same as in Example 1
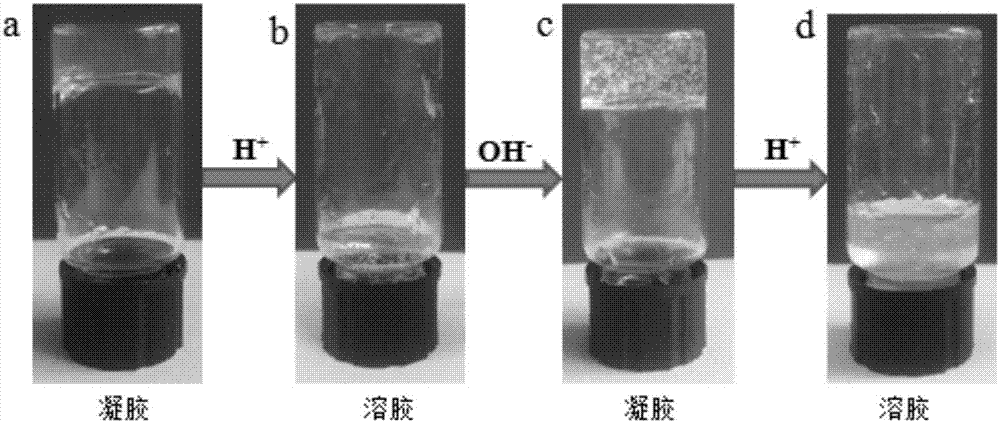
[0032] See figure 2 , the hydrogel ( figure 2 a), add high concentration hydrochloric acid solution (8M, 100μL) to the gel, and shake it on a vortex shaker, the gel will become a sol within 10min ( figure 2 b). Then, an equimolar amount of sodium hydroxide solution (8M, 100 μL) was added to the sol, and the sol transformed into a gel state again within 3 min ( figure 2 c), then add a high-concentration hydrochloric acid solution (8M, 100 μ L), and convert it into a sol ( figure 2 d).
Embodiment 3
[0034] 1) The preparation process and raw materials of multiple responsive polysaccharide hydrogels are the same as in Example 1
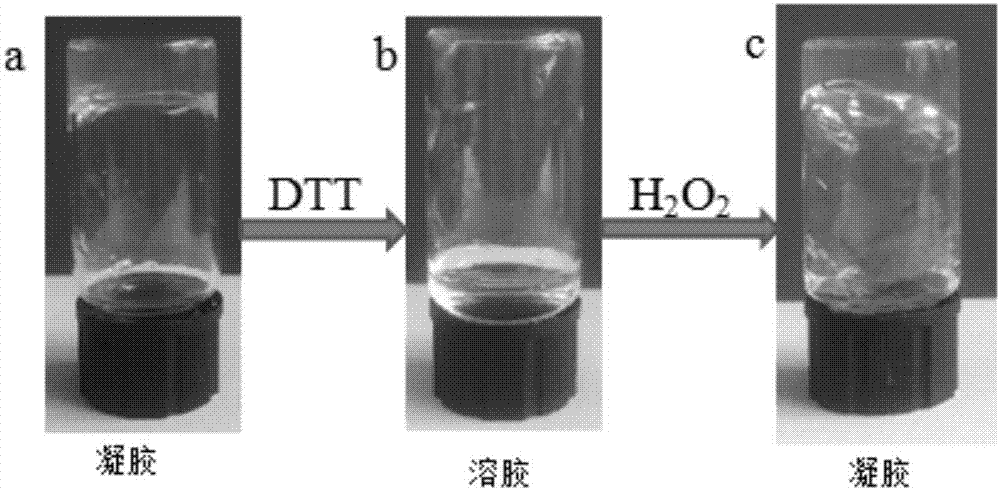
[0035] See image 3, the hydrogel in Example 1 ( image 3 a), add 100 μL, 10wt% DTT solution to the gel, shake and mix evenly on a vortex shaker to realize the transformation from gel to sol ( image 3 b), the required time is 30-60min, then add equimolar aqueous hydrogen peroxide solution (30wt%) in the gained sol, realize from sol to gel ( image 3 c), the required time is 10-30min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com