Crystal forms of flurbiprofen sodium and preparation method thereof
A technology of flurbiprofen sodium and flurbiprofen, which is applied in the pharmaceutical field, can solve problems such as the influence of drug properties, low purity of flurbiprofen sodium, etc., and achieves high liquid phase purity, less types of impurities, and improved stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
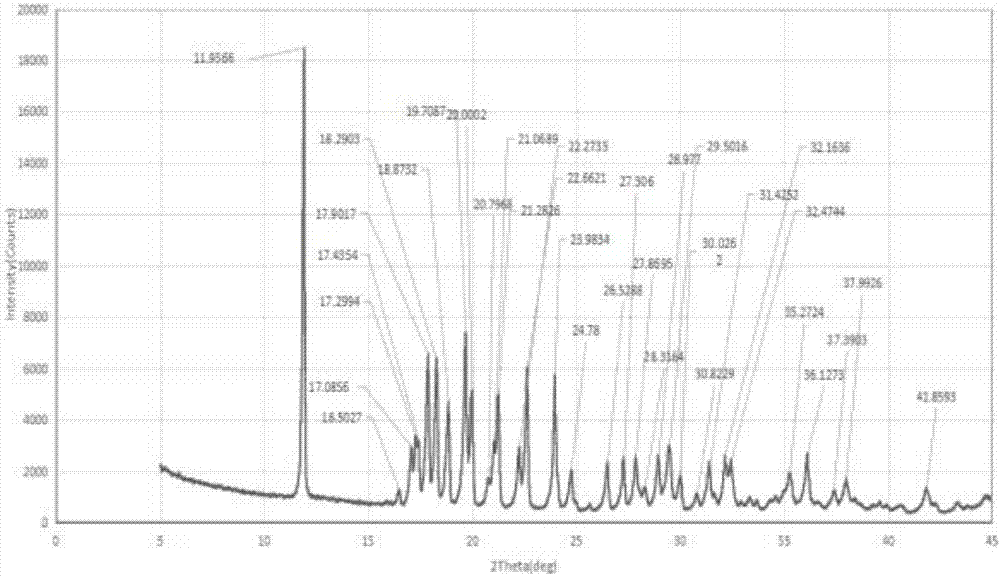
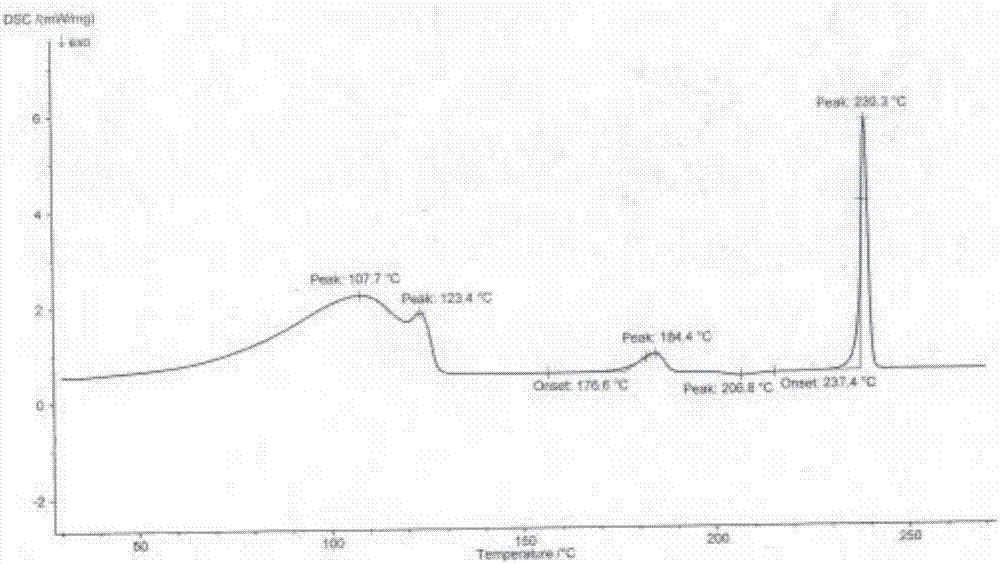
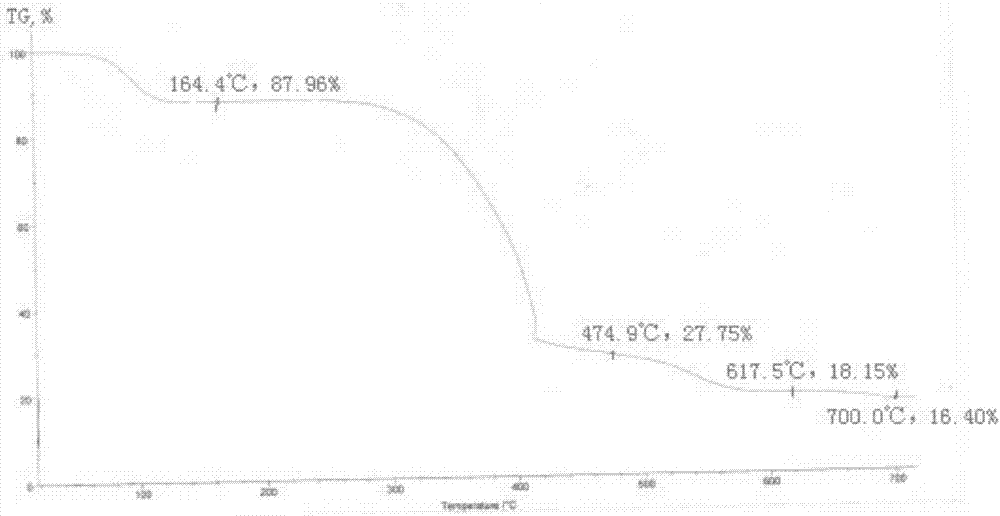
[0047] The preparation method of the flurbiprofen sodium crystal form I is as follows: mix and stir flurbiprofen, an antioxidant and a reaction solvent and heat up to 50-100° C. to dissolve, and the addition amount of the antioxidant is flurbiprofen 0.03%~0.3% of the amount, the proportioning of flurbiprofen and the reaction solvent is 1g:2~5ml, then dropwise add NaOH aqueous solution and stir to react for 0.2~2h, the molar feeding ratio of flurbiprofen and sodium hydroxide 1:1~1:1.2, after stopping the reaction, slowly cool to room temperature, then cool down to -5~15°C, stir and crystallize for 0.5~5h, filter, and put the obtained solid in a vacuum degree of 0.08~0.095Mpa, temperature 25~35 After drying at ℃ for 4-6 hours, the crystal form I of flurbiprofen sodium is obtained in the form of white powder. Wherein, the antioxidant is ascorbic acid or sodium sulfite; the reaction solvent is a single solvent water or a mixture of water and an organic solvent, and the organic sol...
Embodiment 1
[0054] This example provides the preparation process of flurbiprofen sodium crystal form I: add 1.221kg flurbiprofen, 3.7g ascorbic acid, 1.832kg water, 1.447kg ethanol to the reaction kettle, heat up to 70-75°C under stirring Dissolve and clear; then start to drop 30% NaOH aqueous solution (containing NaOH 0.200kg); after dropping, keep stirring at the temperature for 1.5h; stop the reaction, slowly cool to room temperature, then cool down to 0-5°C and stir for 2h to crystallize ; Filtration, the obtained solid was kept in a vacuum greater than 0.08Mpa, dried at a temperature of 30-35°C for 5h, and 1.446kg of a white powdery solid was obtained, which was flurbiprofen sodium crystal form Ⅰ. After testing, the yield of flurbiprofen sodium crystal form I in this example is 95.7%, the liquid phase purity is 99.89%, and the water content is 11.89%, and the obtained flurbiprofen sodium crystal form I has high purity and only contains impurities A, impurity A content is 0.06%.
[0...
Embodiment 2
[0067] This example provides the preparation process of flurbiprofen sodium crystal form II: add 1.221kg flurbiprofen and 2.894kg methanol to the reaction kettle, heat up to 65°C under stirring until dissolved; then start to drop 28% methanol Sodium methanol solution 0.965kg; after dripping, keep the temperature and stir for 1.5h; stop the reaction, slowly cool to room temperature, then cool down to 0-5°C and stir for 2h, filter, and keep the vacuum degree of the obtained solid greater than 0.08Mpa, Dry at a temperature of 30-35°C for 5 hours to obtain 1.262 kg of white powdery solid, which is flurbiprofen sodium crystal form II. After testing, the yield of flurbiprofen sodium crystal form II in this embodiment is 94.8%, the liquid phase purity is 99.55%, and the moisture content is 0.15%, and the obtained flurbiprofen sodium crystal form II has high purity, wherein It only contains impurity A and impurity B, the content of impurity A is 0.31%, and the content of impurity B is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com