Benzoxazole-containing ketopiprazine derivatives as well as synthetic method and application thereof
A technology of benzoxazole piperazinone and synthetic method, which is applied in the field of chemical synthesis containing benzoxazole piperazinone derivatives and their fast and high selectivity, and can solve the problems that are unfavorable to benzoxazole piperazine The application of quasi-derivatives and their industrial synthesis and industrialization are difficult to apply on a large scale, and the operation and post-processing are cumbersome, so as to achieve the effects of low cost, fast response and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0088]
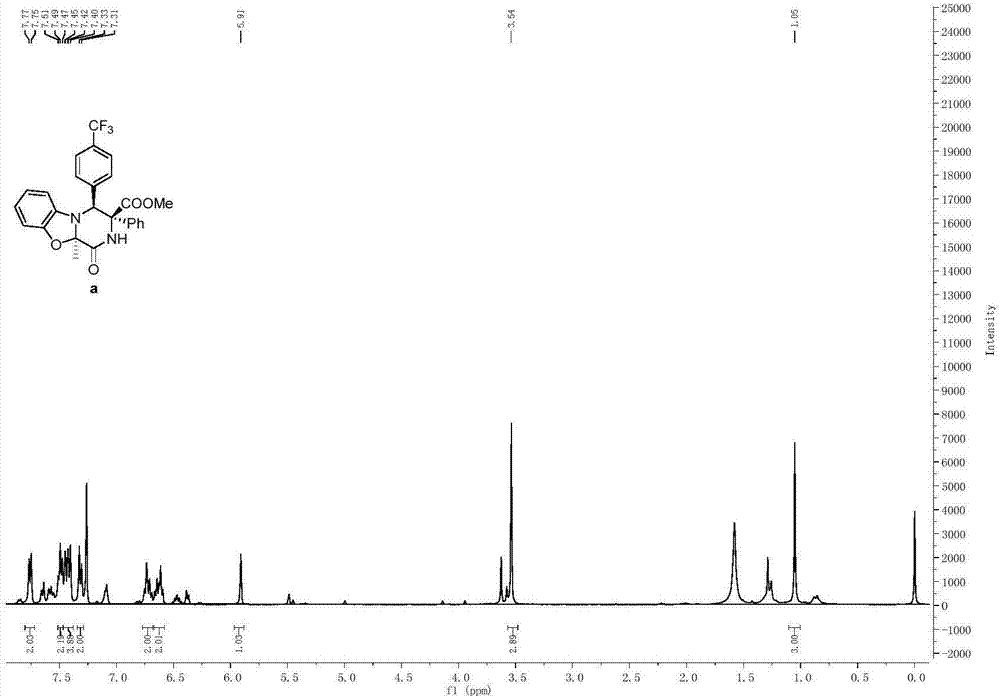
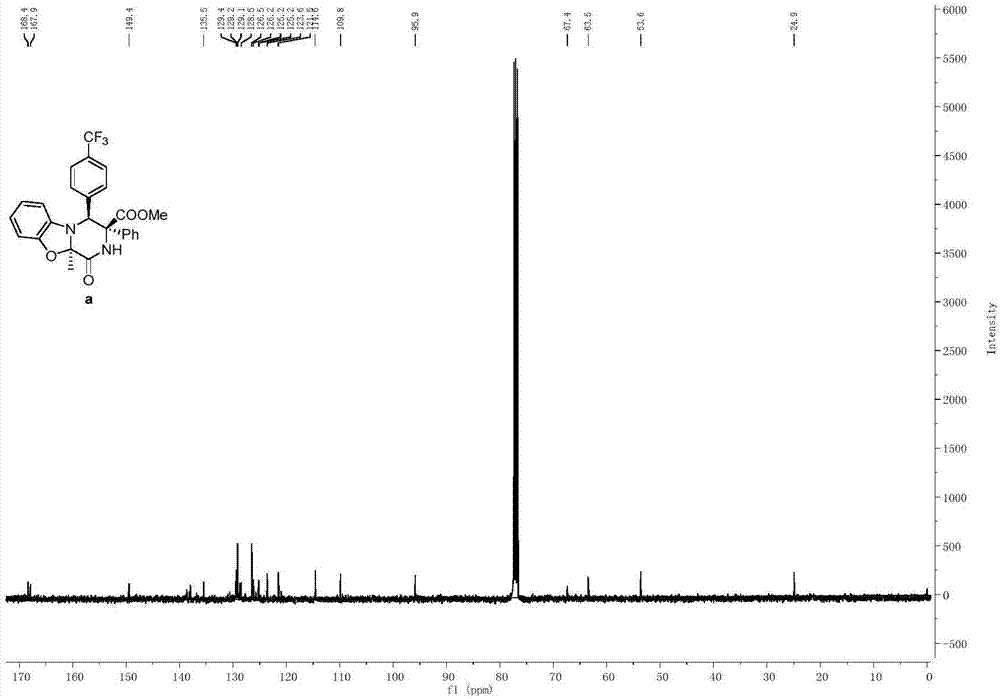
[0089] Propioyl diamino ester compound (0.2mmol) and Molecular sieves (300 mg) were mixed in 2 mL of DCM solvent, and silver trifluoromethanesulfonate (0.04 mmol) was added under nitrogen protection at 0° C., and the reaction was continued for 1 h. After 1 h, the temperature was raised to room temperature (25° C.), and stirring was continued for 2 h. The reaction mixture was purified by column chromatography to obtain a pure product whose structure is shown in formula (a) as methyl-10a-methyl-1-oxo-3-phenyl-4-(4-(trifluoromethyl yl)phenyl)-1,3,4,10a-tetrahydro-2H-benzo[4,5]oxazolone[3,2-a]pyrazine-3-carboxylate. The yield was 65%, and the dr value was equal to >95:5. Compound shown in formula (a) 1 H NMR schematic as figure 1 As shown, its 13 C NMR schematic as figure 2 As shown, its 19 F NMR schematic as image 3 shown.
[0090] 1 H NMR (400MHz, CDCl 3 )δ7.76(d, J=7.7Hz, 2H), 7.49(t, J=7.3Hz, 2H), 7.43(m, 4H), 7.32(d, J=8.1Hz, 2H), 6.72(dd, J=18.1,7....
Embodiment 14
[0092]
[0093] Propioyl diamino ester compound (0.2mmol) and Molecular sieves (300 mg) were mixed in 2 mL of DCM solvent, and silver trifluoromethanesulfonate (0.04 mmol) was added under nitrogen protection at 0° C., and the reaction was continued for 1 h. After 1 h, the temperature was raised to room temperature, and stirring was continued for 2 h. The reaction mixture was purified by column chromatography to obtain a pure product whose structure is shown in formula (b) as methyl-10a-methyl-1-oxo-3-phenyl-4-(4-bromophenyl) -1,3,4,10a-tetrahydro-2H-benzo[4,5]oxazolone[3,2-a]pyrazine-3-carboxylate. The yield was 60%, and the dr value was equal to >95:5. Compound shown in formula (b) 1 H NMR schematic as Figure 4 As shown, its 13 CNMR schematic diagram as Figure 5 shown.
[0094] 1 H NMR (400MHz, CDCl 3 )δ7.67(d, J=7.5Hz, 2H), 7.43-7.37(m, 3H), 7.19(m, 2H), 6.99(d, J=8.1Hz, 3H), 6.69-6.61(m, 2H ),6.61-6.50(m,2H),5.73(s,1H),3.46(s,3H),0.97(s,3H). 13 C NMR (100MH...
Embodiment 15
[0096]
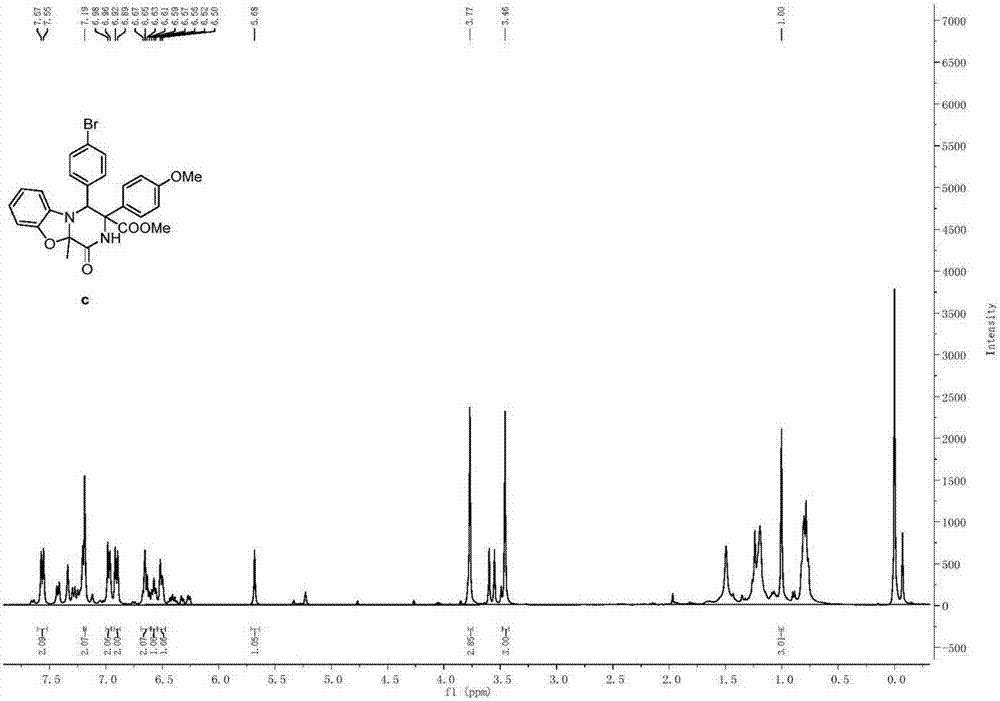
[0097] Propioyl diamino ester compound (0.2mmol) and Molecular sieves (300 mg) were mixed in 2 mL of DCM solvent, silver trifluoromethanesulfonate (0.04 mmol) was added under nitrogen protection at 0°C, and the reaction was continued for 1 h. After 1 h, the temperature was raised to room temperature, and stirring was continued for 2 h. The reaction mixture was purified by column chromatography to obtain a pure product whose structure is shown in formula (c) as methyl-10a-methyl-1-oxo-3-(4-methoxyphenyl)-4- (4-Bromophenyl)-1,3,4,10a-tetrahydro-2H-benzo[4,5]oxazolone[3,2-a]pyrazine-3-carboxylate. The yield was 50%, and the dr value was equal to >95:5. Compound shown in formula (c) 1 H NMR schematic as Image 6 As shown, its 13 C NMR schematic as Figure 7 shown.
[0098] 1 H NMR (400MHz, CDCl 3 )δ7.56(d, J=8.7Hz, 2H), 7.19(s, 2H), 6.97(d, J=8.3Hz, 2H), 6.91(d, J=8.7Hz, 2H), 6.64(q, J=7.6Hz, 2H), 6.57(t, J=7.2Hz, 1H), 6.51(d, J=7.1Hz, 2H), 5.68(s, 1H), 3.77...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com