Biflavone-manganese complex, as well as preparation method and application thereof
A technology of manganese complexes and biflavones, which is applied in the field of bisflavone-manganese complexes and its preparation, can solve the problems that the synthesis and biological activity of bisflavone complexes have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
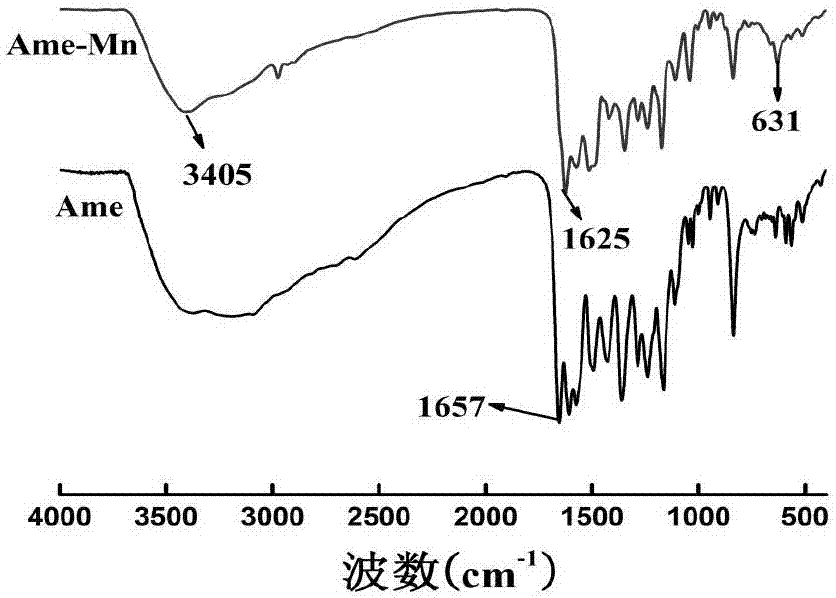
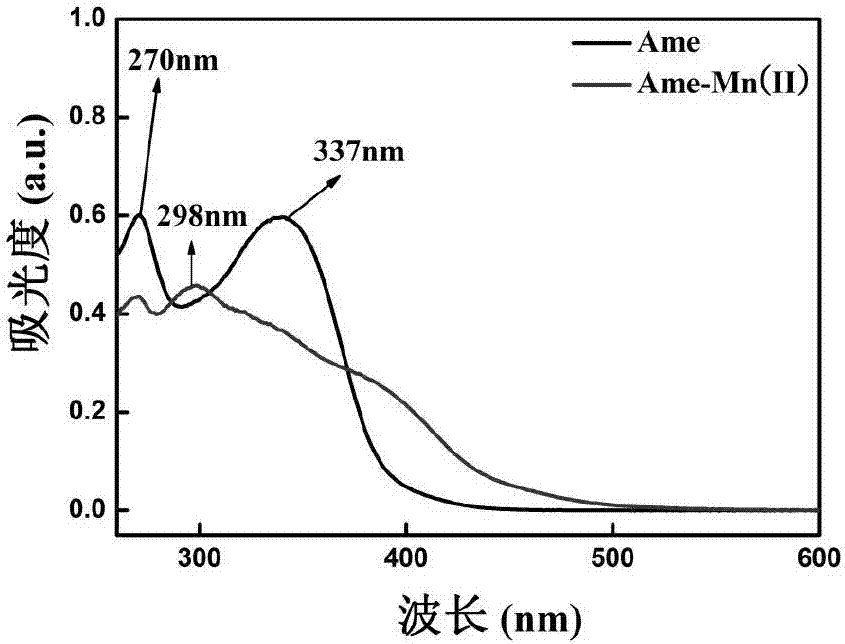
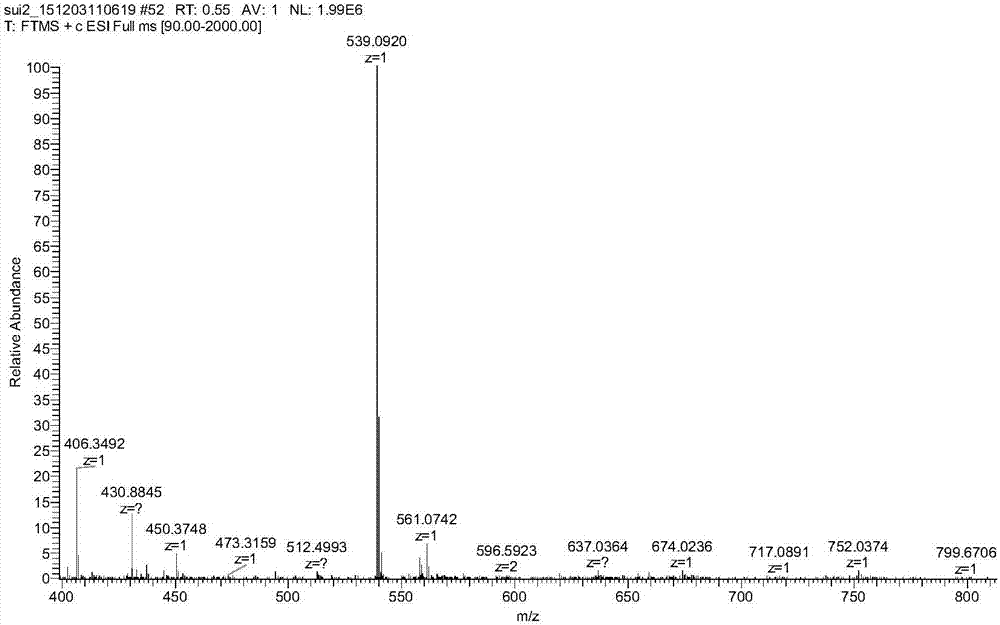
[0039] Accurately weigh 53.8mg of Ame in a round bottom flask, dissolve it with 5mL of ethanol, accurately weigh 17.9mg of 50% manganese nitrate solution (containing 0.05mmol of manganese nitrate), add it to 5mL of ethanol, add the manganese nitrate solution dropwise to the Ame solution , add ethanol-ammonia water (V / V, 3:1) solution dropwise to the reaction solution, adjust the pH to 6, keep it at 30°C for 4-5h, precipitate occurs, filter, wash with ethanol and water in turn, and recrystallize with DMSO , freeze-dried to obtain the Ame-Mn complex.
Embodiment 2
[0041] Accurately weigh 53.8 mg of Ame in a round bottom flask, dissolve it with 5 mL of 90% ethanol, accurately weigh 17.9 mg of 50% manganese nitrate solution (containing 0.05 mmol of manganese nitrate), add it to 5 mL of 90% ethanol, and drop the manganese nitrate solution Add it to the Ame solution, add ethanol-sodium ethylate solution dropwise to the reaction solution, adjust the pH to 5, keep it at 30°C for 4-5h, a precipitate occurs, filter, wash with ethanol and water in turn, recrystallize in DMSO, and freeze-dry to obtain Ame-Mn complexes.
Embodiment 3
[0043] Accurately weigh 53.8mg of Ame in a round bottom flask, dissolve it with 5mL of methanol, accurately weigh 17.9mg of 50% manganese nitrate solution (containing 0.05mmol of manganese nitrate), add it to 5mL of methanol, and add the manganese nitrate solution dropwise to Ame In the solution, add methanol-sodium methoxide solution dropwise to the reaction solution, adjust the pH to 7, keep at 40°C for 3-4h, precipitate occurs, filter, wash with methanol and water successively, recrystallize in DMSO, and freeze-dry to obtain Ame-Mn Complexes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com