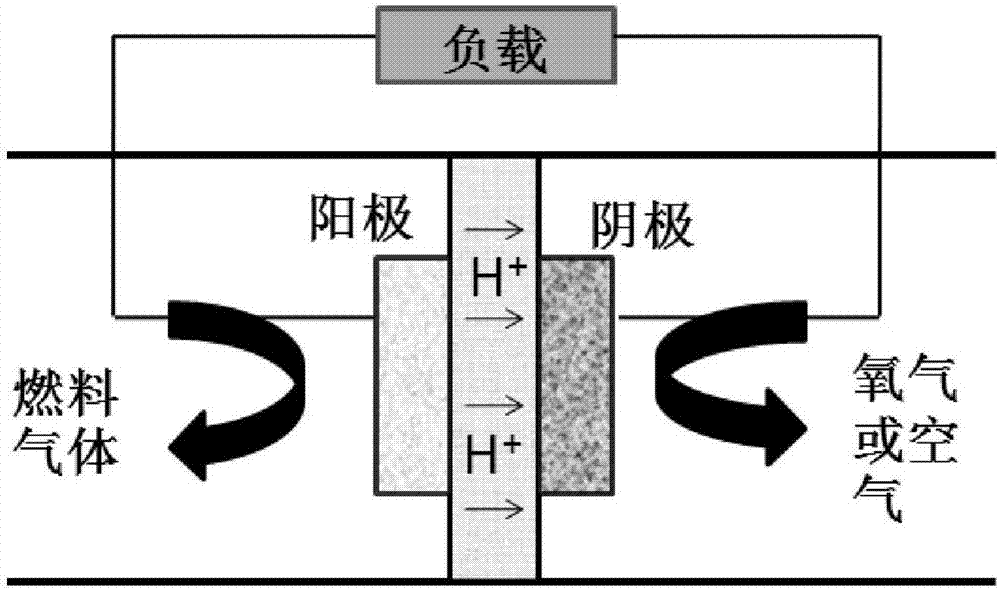
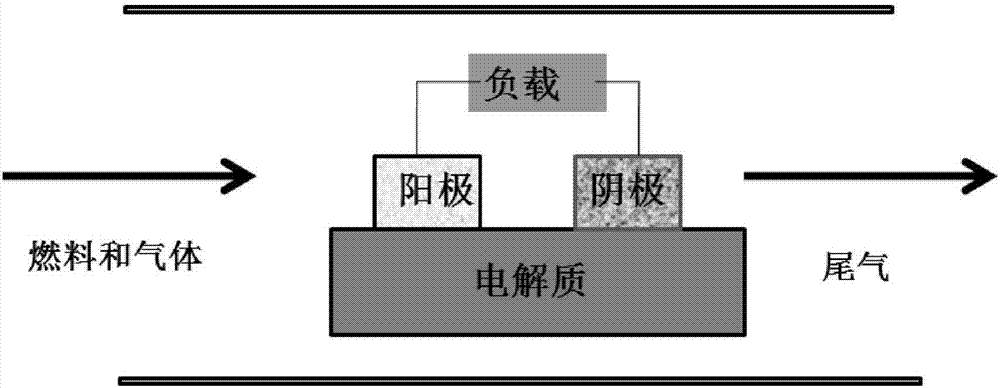
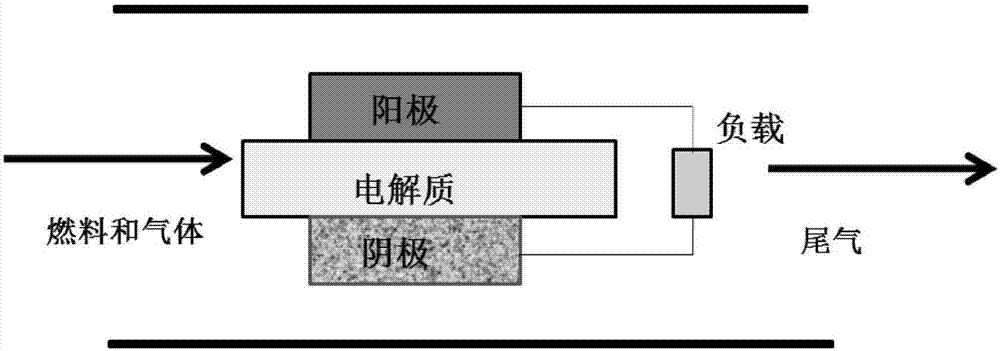
High-temperature ionic liquid-based fuel cell
A technology for ionic liquids and fuel cells, applied in fuel cells, fuel cell parts, battery electrodes, etc., can solve the problems of easy poisoning of catalysts, high cost, easy loss of proton conductors, etc., and achieve the effect of preventing electrodes from being flooded
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] This embodiment provides an electrolyte-supported fuel cell named SDC-IL battery, the electrolyte diaphragm in the fuel cell is a solid oxide / ionic liquid composite electrolyte diaphragm, and the solid oxide is an oxygen ion type solid oxide SDC (the solid oxide / ionic liquid composite electrolyte membrane is referred to as SDC-IL composite electrolyte membrane for short).
[0100] Preparation:
[0101] (1) Synthesize SDC powder by urea spontaneous combustion method. The molar ratio of metal ions to urea is 1:3, the powder obtained by spontaneous combustion is ball-milled with alcohol for 48 hours, and the powder obtained after drying is pressed into a small cake with a diameter of 20mm and a thickness of 1mm under a pressure of 100Mp, and then Sinter at 1000°C for 4h.
[0102] (2) Preparation of SDC-IL composite electrolyte separator: SDC was used as a substrate, and ionic liquid (the ionic liquid was nitrogen methylimidazole trifluoromethanesulfonate) was vacuum impr...
Embodiment 2
[0109] This embodiment provides an anode-supported fuel cell named (NiO+SDC)-(SDC-IL) cell, the electrolyte membrane in the fuel cell is a solid oxide / ionic liquid composite electrolyte membrane, and the solid The oxide is an oxygen ion type solid oxide SDC (the solid oxide / ionic liquid composite electrolyte membrane is referred to as SDC-IL composite electrolyte membrane for short); the anode material in the fuel cell is a mixture of NiO and SDC.
[0110] Preparation:
[0111] (1) SDC powder was synthesized by co-precipitation method. Weigh a certain amount of samarium nitrate and cerium nitrate according to the stoichiometric ratio and dissolve them in deionized water, then add the obtained nitrate solution back dropwise into ammonia water, heat and stir at 80°C for 2 hours, cool to room temperature, and deionize successively SDC raw powder was obtained after washing with water and alcohol suction filtration and drying, and annealed at 600°C and 800°C for 2 hours respective...
Embodiment 3
[0119] The solid oxide in the solid oxide / ionic liquid composite electrolyte separator is proton-type solid oxide BaCe 0.6 Zr 0.3 Y 0.1 o 3-δ , and replace the SDC substrate with BaCe 0.6 Zr 0.3 Y 0.1 o 3-δ Except for the base, other contents are the same as in Example 1.
[0120] The same method as in Example 1 was used to test, and the result showed that a voltage of 0.9V could be obtained at 300°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com