Medicine composition containing empagliflozin and dimethylbiguanide hydrochloride
A technology of metformin hydrochloride and its composition, which is applied in the field of medicine, can solve problems such as day-to-day blood drug concentration variation, unfavorable coating process, and unqualified friability, and achieve the effects of high hardness, rapid dissolution, and adjustable output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The selection of embodiment 1 prescription quantity
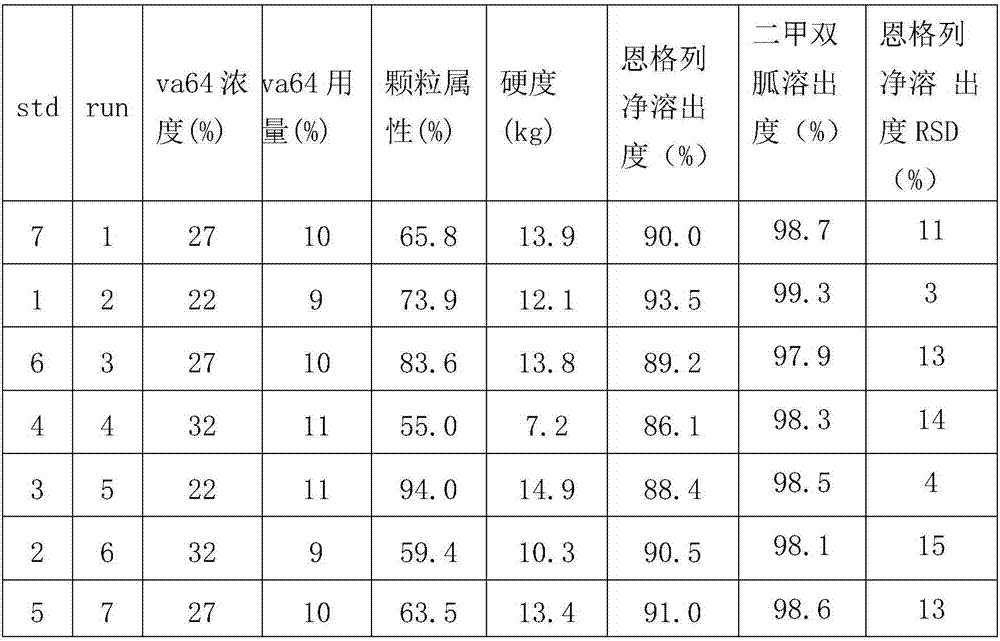
[0029] The amount of excipients in the formulation was determined by the method of Design of Experiments (DOE), and the factors affecting the key quality attributes of the tablet were investigated at the same time. use 2 2 -The full factorial design of three central points, to investigate the effect of binder dosage and binder concentration on particle properties (the weight of particles passing through 24 mesh sieve but not 100 mesh sieve), hardness and dissolution rate. The experimental combination and results are shown in Table 1.
[0030] Table 1 Amount and concentration of excipients 2 2 -Three center point full factorial design table and results
[0031]
[0032] The specific amount of raw and auxiliary materials for each combination is shown in Table 2
[0033] Table 2 The amount of raw and auxiliary materials used in each combination
[0034] Dosage (g) std 1 std 2 std 3 std 4 std 5...
Embodiment 2
[0038] The selection of embodiment 2 adhesive types
[0039] When adopting the granulation process, the type of adhesive will have a greater impact on the dissolution of the tablet. The present invention has carried out a screening test on the type of adhesive. The experimental combination and results are shown in Table 3-4
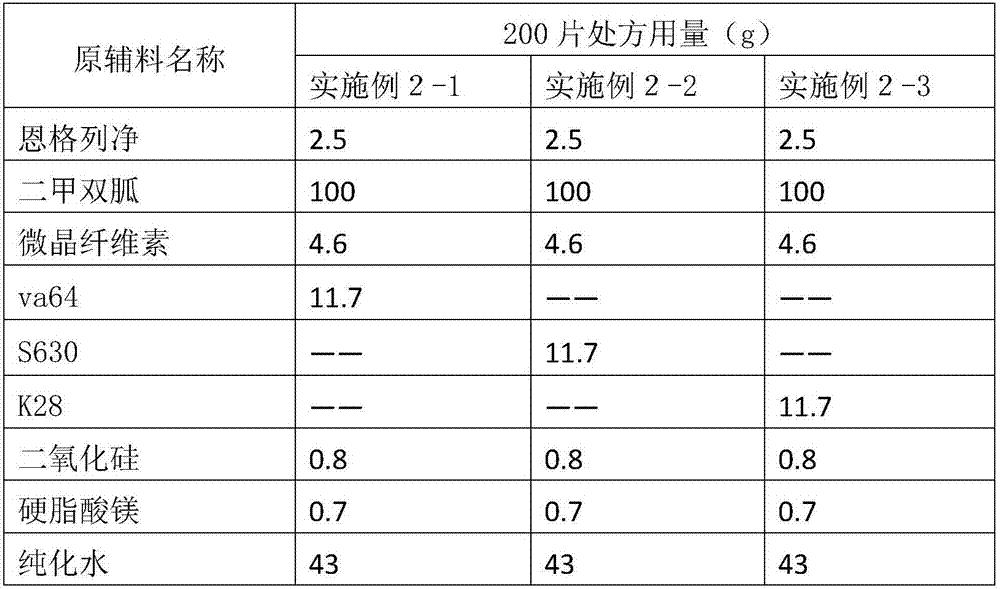
[0040] Table 3 composition of different adhesive formulations
[0041]
[0042] Table 4 The results of the investigation on the impact of the type of binder on the key attributes of the tablet
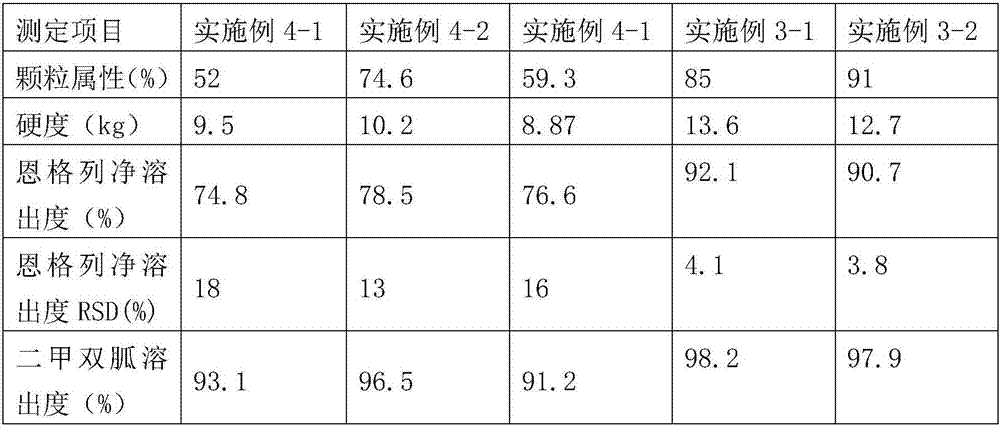
[0043] Measurement items Example 2-1 Example 2-2 Example 2-3 Particle properties (%) 90 67 65 Hardness (kg) 12.1 7.0 8.9 Dissolution of Empagliflozin (%) 94.8 98.6 76.6 Dissolution rate of metformin (%) 101.1 100.5 89.2 Dissolution RSD of Empagliflozin (%) 3.4 12 14
[0044] According to the above test results, va64 is more suitable for the completion of the present invention than other types of adhes...
Embodiment 3
[0045]Embodiment 3 Concrete prescription
[0046] See Table 5 for the composition of single-layer tablets (cores) of Empagliflozin, Metformin Hydrochloride 5mg / 500mg and 12.5mg / 500mg.
[0047] Table 5 Prescription
[0048] Embodiment 3-1 (10,000 pieces) Feed amount (g) weight% Empagliflozin 50 0.85 Metformin 5000 84.75 microcrystalline cellulose 153.1 2.6 va64 620 10.5 silica 41.4 0.7 Magnesium stearate 35.5 0.6 purified water 2700L (va64 concentration) 23
[0049] Embodiment 3-2 (10,000 pieces) Feed amount (g) weight% Empagliflozin 125 2.12 Metformin Hydrochloride 5000 84.75 Microcrystalline Cellulose PH101 167.1 2.83 va64 531 9.00 Colloidal silica 41.4 0.70 Magnesium stearate 35.5 0.60 purified water 2140L (va64 concentration) 24.8
[0050] Preparation operation:
[0051] Metformin hydrochloride and microcrystalline cellulose, empagliflozi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com